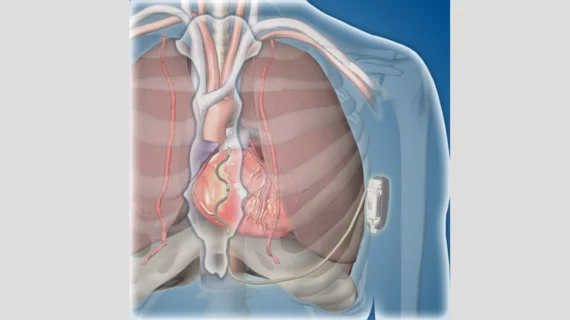
Medtronic has gained European CE mark approval for its Aurora EV-ICD MRI SureScan and Epsila EV MRI SureScan defibrillation lead, which are designed to help treat severe heart rhythm issues.
The Aurora EV-ICD system is unlike most other implantable cardioverter-defibrillators (ICDS), the company said in a prepared statement, because the lead is placed outside the patient’s heart and veins. Instead, the lead is placed under the patient’s breastbone; this is intended to help clinicians avoid long-term complications such as vessel occlusion, blood infections or the need for intravenous lead extraction. The lead is then connected to a device implanted below the patient’s left armpit.
Other features of the newly approved solution include anti-tachycardia pacing, pause prevention pacing and Medtronic’s PhysioCurve design, which aims to improve patient comfort.
“We are proud to be the first company to offer a complete one-system, one-procedure extravascular ICD solution, which maintains the patient benefits of traditional, transvenous ICDs without the risk of leads in the heart and vasculature,” Alan Cheng, MD, chief medical officer of Medtronic’s cardiac rhythm management business, said in the statement. “This approval is a significant milestone in achieving our goal of delivering a defibrillation solution that treats sudden cardiac arrest while improving the patient experience.”
“The growing awareness by patients and physicians about the risks that come with placing leads in the heart or veins is addressed by the Aurora EV-ICD system that provides an extravascular solution while maintaining the traditional ICD benefits of pacing and defibrillation therapy,” added cardiologist Lucas V.A. Boersma, MD, PhD, a heart failure and arrhythmia specialist with Amsterdam University Medical Centers in the Netherlands.
CE mark approval was based in part on data taken from the Medtronic-funded Extravascular ICD Pivotal Study; findings from that analysis were published in The New England Journal of Medicine in October 2022.
“In this prospective global study, we found that extravascular ICDs were implanted safely and were able to detect and terminate induced ventricular arrhythmias at the time of implantation,” the study’s authors wrote at the time.
In the United States, the Aurora EV-ICD system is still just being used for investigational purposes. It has not been approved for commercial use by the U.S. Food and Drug Administration.