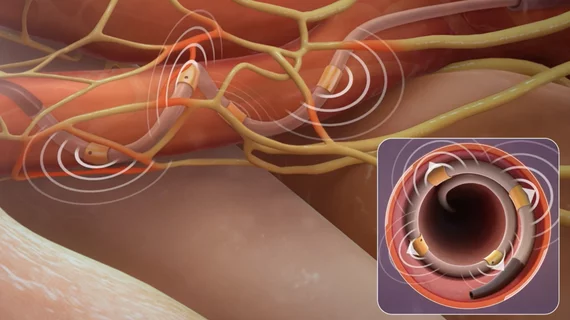
The SPYRAL HTN-ON MED renal denervation (RDN) trial failed to show that renal denervation was superior to sham control at improving ambulatory blood pressure (ABPM), but there was a modest improvement in office blood pressure. These were the key findings in the first six months of data of the full trial enrollment data presented as a late-breaking study at the American Heart Association (AHA) 2022 meeting.
An America College of Cardiology (ACC) review of the data noted the trial did not meet its endpoint, but did note the modest improvement in office blood pressure and this translating into lower amounts of medication being prescribed. This was played up during the AHA presentation and by the study sponsor Medtronic.
"The ON MED study demonstrated significant reductions in office-based blood pressure, the most commonly used measure in clinical practice. Additionally, we saw reductions in absolute blood pressure that were consistent with earlier RDN studies," said David Kandzari, MD, chief, Piedmont Heart Institute and Cardiovascular Services and SPYRAL HTN-ON MED lead principal investigator, in a statement on the data.
Kandzari said the reasons for this trial possibly not meeting the primary endpoint may have been due to impacts from COVID-19.
"Surprisingly, 24-hour ABPM declined with RDN, but did not differ from the sham group, and the primary endpoint was not met," he said in the statement.
More than 80% of patients in the ON MED expansion group did go through followup during the COVID-19 pandemic. Based on this data, it appears the difference in outcomes from previous positive SPYRAL trials may be due to changes in the patients' lives during COVID.
"Compared with patients enrolled before the pandemic, significant differences in baseline 24-hour ABPM were observed that may reflect changes in patient behavior and lifestyle during the pandemic. Additionally, patients treated with the sham procedure increased the amount of medication they were taking compared to those treated with RDN. These factors likely contributed to the smaller-than-expected differences in ABPM," Kandzari explained.
Medtronic said with this additional data, it has submitted the final module of the Symplicity Spyral premarket approval (PMA) application to the U.S. Food and Drug Administration (FDA) for final review.
In the trial, patients who were prescribed antihypertensive medications and treated with the Medtronic Symplicity Spyral Renal Denervation System had a statistically significant and clinically meaningful reduction in office-based systolic blood pressure (OSBP), a key secondary endpoint, compared to subjects in the sham control group.
Details from the SPYRAL HTN-ON MED trial
SPYRAL HTN-ON MED is a global, randomized, sham-controlled trial investigating the blood pressure lowering effect and safety of RDN with the radiofrequency (RF)-based Symplicity Spyral RDN system in hypertensive patients who have been prescribed up to three anti-hypertensive medications, including diuretics, calcium channel blockers, ACE/ARB inhibitors or beta blockers. A total of 337 patients with uncontrolled hypertension were enrolled at 42 sites across the United States, Europe, Japan, Australia and Canada, and were randomized 2:1 to RDN (n=205) versus sham control (n=132).
Key findings include:
• The primary Bayesian efficacy endpoint of 24-hour systolic ABPM reduction was not met, with a 51% probability of superiority for the RDN group versus those who received a sham control procedure. However, nighttime systolic ABPM reduction was statistically significant.
• 6.5 mmHg 24-hr systolic ABPM reduction in the RDN group versus 4.5 mmHg in the control group (treatment difference of -1.9 mmHg, p=0.119).
• 6.7 mmHg nighttime systolic ABPM reduction in the RDN group versus 3 mmHg in the control group (treatment difference of -3.7 mmHg, p=0.01).
• The study met the prespecified secondary endpoint, which was the change in OSBP from baseline to six-month follow-up between the RDN group (n=199) and the sham control group (n=126).
Statistically significant 9.9 mmHg OSBP reductions in the RDN group versus a 4.9 mmHg reduction in the sham control group (treatment difference of -4.9 mmHg, p=0.001).
• The study met the primary safety endpoint, evaluating major adverse events at one-month post-procedure, and renal artery stenosis at six-months, pooled across the SPYRAL HTN-ON and OFF MED studies (p<0.001).
• RDN demonstrated a low incidence of procedure-related and clinical adverse events at six-months in the SPYRAL HTN-ON MED study specifically.
• A Win Ratio analysis demonstrated significance in favor of RDN versus a sham procedure (p=0.005).
• Overall burden of medications was higher in the sham control group at six-months (p=0.04).
The SPYRAL HTN-ON MED Trial is a part of the Medtronic SPYRAL HTN Global Clinical Program, which includes the SPYRAL HTN-OFF MED Pivotal Trial and the currently-enrolling SPYRAL AFFIRM Study. Along with the real-world data from the Global SYMPLICITY Registry (GSR), Medtronic says it now has more than 20,000 patients treated globally, studied in the presence and absence of medication.
The Symplicity Spyral RDN system is approved for commercial use in more than 60 countries around the world. It is limited to investigational use in the United States, Japan and Canada.