Cooling device now approved by FDA to reduce risk of ablation-related esophageal injuries
Attune Medical, the Chicago-based healthcare technology company, has received a De Novo approval from the U.S. Food and Drug Administration (FDA) for its ensoETM device to limit the risk of esophageal injury during radiofrequency cardiac ablation procedures.
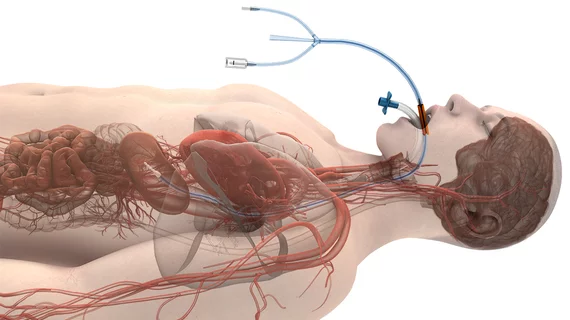
The ensoETM device is a single-use silicone tube placed in the patient’s esophagus, much like a traditional orogastric tube, and then connected to an external heat exchange unit. This creates a closed loop, allowing water to circulate and cool or warm the patient as needed. While originally approved by the FDA to control patient temperature, it is now officially approved to reduce the risk of esophageal injuries, a known side effect of radiofrequency ablation that can put a patient’s life in jeopardy.
The FDA’s decision was in part due to the success seen in the eCOOL-AF and IMPACT studies, which included evidence that using the ensoETM device is associated with fewer esophageal injuries and fewer cases of atrioesophageal fistula.
“Over the last 20 years, significant resources have been committed to mitigating serious esophageal complications, with no meaningful results,” Jay Istvan, Attune Medical CEO, said in a prepared statement. “This De Novo marketing authorization opens the door to a new standard. Studies have shown improved safety and efficacy for patients while allowing improved efficiency for physicians and greater cost savings for the hospitals in which they operate.”
“Historically, there have been no proven strategies to prevent esophageal injury during ablation procedures, and injury rates have not declined despite the use of temperature probes,” added Jason Zagrodzky, MD, an electrophysiologist at Texas Cardiac Arrhythmia in Austin, Texas. “This De Novo authorization gives electrophysiologists a solution to proactively cool the esophageal wall during ablation procedures and is a great leap forward in best practice standards and patient care.”