Heart failure specialists raise $58M for new device designed to improve cardiac, renal function
Procyrion, a Houston-based healthcare technology company, has raised $57.7 million in Series E funding to support ongoing research into its Aortix percutaneous mechanical circulatory support (pMCS) device.
A majority of the money will help Procyrion complete the DRAIN-HF clinical trial, which is evaluating the safety and effectiveness of the Aortix pMCS device in acute decompensated heart failure (ADHF) patients with cardiorenal syndrome (CRS) compared to diuretics alone. Any additional funds will go toward fine-tuning the manufacturing process and planning for the eventual commercialization of the new-look ADHF offering.
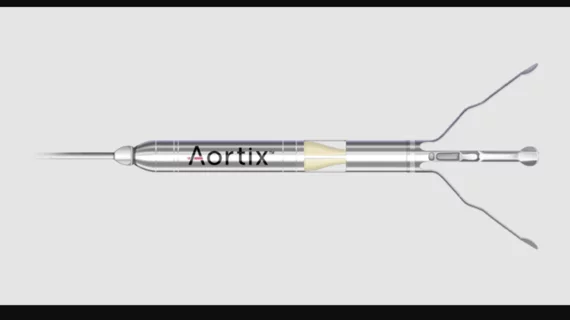
The Aortix pMCS device is implanted into a patient’s descending thoracic aorta to increase perfusion to the kidneys, which can increase urine output, and unload the heart. It was designed to pump blood without the need of a heart valve. Early data, published in JACC: Heart Failure in November 2023, suggest treatment with the device is associated with rapid decongestion, improved hemodynamics and other key clinical benefits for up to 30 days.[1]
“Approximately 25% of the millions of patients admitted to the hospital with ADHF are unable to be successfully treated with standard-of-care therapies, yet there is a lack of effective treatment options available, leading to very poor outcomes,” Eric S. Fain, MD, president and CEO of Procyrion, said in a statement announcing the news. “Aortix therapy is uniquely suited for treating CRS patients and this latest round of investment will enable the company to make significant progress toward commercialization of our technology. We thank our investors for recognizing the engineering and clinical achievements to date, as well as the potential of Aortix to be a truly groundbreaking advancement to break the vicious CRS cycle and improve the outcomes in these most challenging-to-manage heart failure patients who currently have no proven therapeutic options.”
Fannin Partners teamed with a series of new and existing investors to lead the Series E financing round. Returning investors, including Bluebird Ventures, also participated.
“We are pleased to lead this round as we see enormous commercial potential for Aortix and look forward to seeing the results of the DRAIN-HF study with the goal of demonstrating improved patient outcomes, which should also reduce the burden on the overall healthcare system,” added Leo Linbeck III, chairman and founder of Fannin Partners and a Procyrion board member.