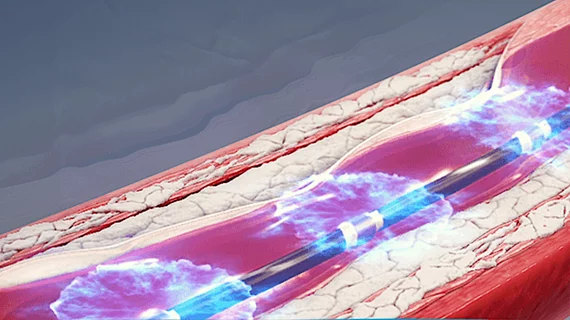
IVL Turns Standard Balloon Technology into a Powerful Calcium-disruption Tool
Can a disruptive technology from the past become the nemesis of calcified lesions in the future?
Proponents of intravascular lithotripsy (IVL), approved by the U.S. Food and Drug Administration in February 2021 for treatment of severely calcified coronary artery disease, respond with a hearty yes. Tools that can make stent delivery and deployment through plaque-laden arteries less taxing and more procedurally effective for operators, they contend, are urgently needed. And research suggests these tools will offer significant benefits for patients.
Intravascular lithotripsy is a welcome disruptor in interventional cardiology
In its latest incarnation, IVL from Shockwave Medical combines a balloon catheter with multiple lithotripsy emitters that fracture intimal and medial calcium in the artery walls through sonic pressure waves that are tissue sensitive and does not require high-pressure balloon inflation.
“The brilliance of Shockwave is that it’s a leading-edge technology packaged and delivered in a primitive device which we as interventionalists use all the time: a balloon,” says Dean Kereiakes, MD, medical director of The Christ Hospital Heart and Vascular Center in Cincinnati, and co-principal investigator of the DISRUPT CAD III study (J Am Coll Cardiol 2020;76[22]:2635-46), which paved the way for FDA approval. “It’s the most reliable, effective, and safe calcium-modifying technology now available, particularly in larger vessels and longer target lesions. That’s why I believe its adoption by the interventional community will be easy and swift.”

The Shockwave device was first FDA approved in 2016 for treating patients with peripheral artery disease (PAD). The safety and utility of that application was reinforced by DISRUPT PAD III (J Endovasc Ther 2020;27[3]:473-80), which found IVL to be superior to percutaneous transluminal angioplasty in the treatment of calcified femoral popliteal disease. When compared with percutaneous transluminal angioplasty, IVL was further associated with a statistically significant reduction in diameter stenosis, arterial dissections and bail-out stenting.
Will the hype be right for Intravascular lithoplasty?
Coronary artery calcium modification is what now has the field abuzz, however. IVL (variously known as lithotripsy or lithoplasty) is already leaving a successful imprint in Europe and has found increasing usage on both sides of the Atlantic as an alternative access route for TAVR through plaque-heavy iliac arteries.
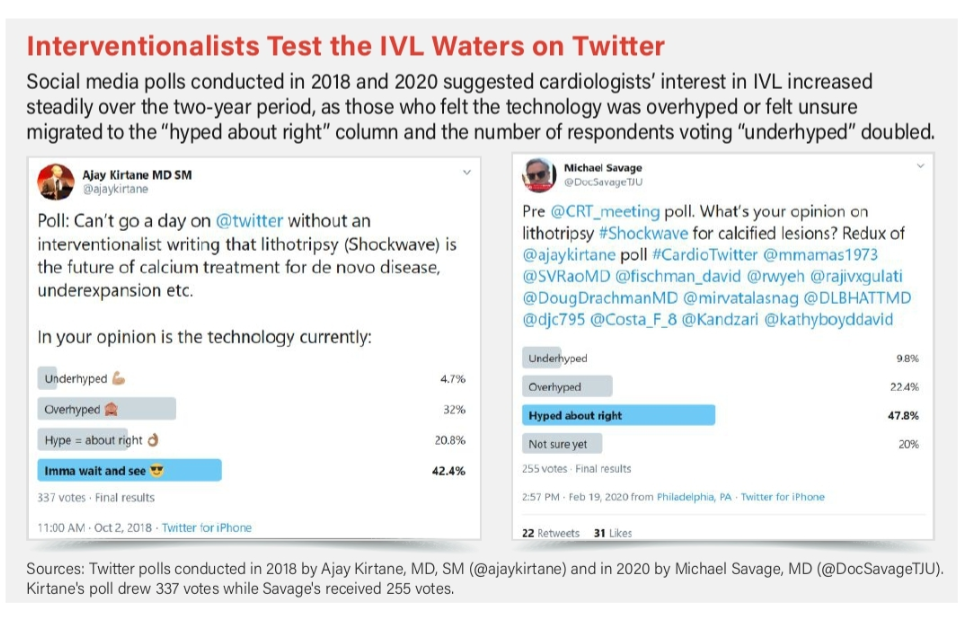 Chatter among interventionalists hints at IVL’s potential. A February 2020 Twitter poll by Michael Savage, MD, director of cardiac catheterization at Thomas Jefferson University Hospital in Philadelphia, showed that nearly 48 percent of responding physicians felt that the hype was “about right,” 22 percent that it was overhyped, nearly 10 percent that it was underhyped and 20 percent weren’t sure. A favorable trendline emerges when compared to a similar Twitter poll posted at the end of 2018 by Ajay Kirtane, MD, SM, associate professor of medicine at Columbia University Medical Center in New York, when over 42 percent were taking a wait-and-see attitude regarding the technology, while 32 percent felt it was overhyped and 21 percent that it was “hyped about right.”
Chatter among interventionalists hints at IVL’s potential. A February 2020 Twitter poll by Michael Savage, MD, director of cardiac catheterization at Thomas Jefferson University Hospital in Philadelphia, showed that nearly 48 percent of responding physicians felt that the hype was “about right,” 22 percent that it was overhyped, nearly 10 percent that it was underhyped and 20 percent weren’t sure. A favorable trendline emerges when compared to a similar Twitter poll posted at the end of 2018 by Ajay Kirtane, MD, SM, associate professor of medicine at Columbia University Medical Center in New York, when over 42 percent were taking a wait-and-see attitude regarding the technology, while 32 percent felt it was overhyped and 21 percent that it was “hyped about right.”
“People in the field are definitely excited about this technology,” says Savage. “The big winner in the cath lab is any device that saves time and is easy to use. And what we’re seeing with Shockwave is familiar balloon technology that goes over a standard wire and simplifies the process. Hopefully, it will give us another tool to effectively tackle hard, calcified lesions that are becoming more and more of a problem.”
Kirtane echoes that thought, citing the utility of a technology that’s “balloon-based with low inflation pressure. If the device can cross by conventional means, there’s theoretically less potential for vessel injury, which is why there’s a lot of interest in intravascular lithotripsy.”
Related Content on Intravascular Lithotripsy to Break Up Calcified Lesions:
VIDEO: Shockwave Medical demonstrates faster intravascular lithotripsy at ACC22
Shockwave’s IVL technology gains FDA approval for treating advanced coronary artery disease
Better Together? An Integrated Market for Calcium-modification Strategies
CMS recommends additional payment for using Shockwave’s IVL technology in hospital setting