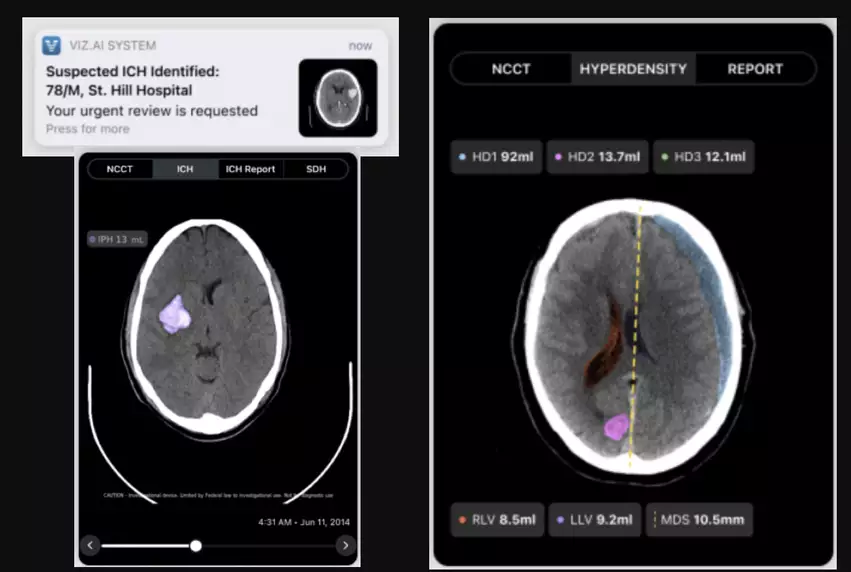
Viz.ai, the San Francisco-based healthcare company focused on artificial intelligence (AI) technologies, has gained U.S. Food and Drug Administration (FDA) approval for an advanced AI model designed to guide the treatment of intracranial hemorrhage (ICH) patients. ICHs, commonly referred to as “bleeding strokes,” can be life-threatening if not treated in a timely manner.
The newly approved algorithm, Viz ICH Plus, is capable of identifying, labeling and quantifying brain bleeds in noncontrast CT images. It can evaluate intracranial hyperdensities, lateral ventricles and midline shift, delivering users with immediate volume measurements. This helps monitor the brain bleed for any changes and guide the care team’s plan moving forward.
“The ability and mobility to obtain accurate and quantifiable measurements of intracerebral hemorrhages through Viz ICH Plus significantly enhances our decision-making process,” Peter Kan, MD, professor and chair of the department of neurosurgery at the University of Texas Medical Branch, said in a statement. “This technology, which marries precision with AI, is poised to transform how we approach intracerebral hemorrhage cases.”
“Viz ICH Plus exemplifies our dedication to enhancing patient care by leveraging technology,” added Jayme Strauss, chief clinical officer at Viz.ai. “We are excited to introduce a product that bridges the gap between AI capabilities and improved patient outcomes.”
Viz.ai, which started as a neurosurgeon-founded startup in 2016, has been a leading player in the AI space for years now. The company’s algorithm for identifying hypertrophic cardiomyopathy (HCM) received a de novo approval from the FDA in August 2023, and the all-in-one Viz.ai Platform now includes more than a dozen different FDA-approved AI models.
According to Viz.ai, more than 1,500 U.S. hospitals are actively using at least one of the AI models available through Viz.ai Platform. The platform runs on its own, assessing medical images as they are captured and alerting physicians immediately when sings of a significant problem are detected.