First personal 6-lead ECG cleared for sale in US
AliveCor has been granted FDA clearance for KardiaMobile 6L, the world’s first publicly available six-lead personal electrocardiogram device.
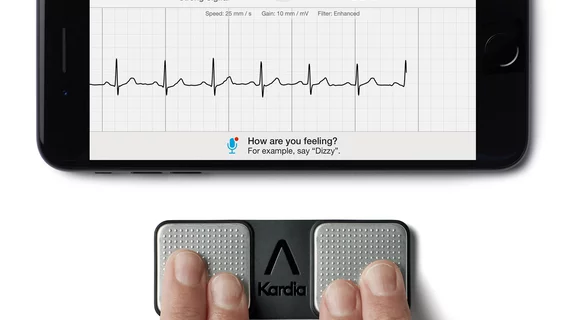
Clearance of the KardiaMobile 6L marks the third time one of AliveCor’s technologies has been approved by the FDA in the space of three months, the company said in a May 13 release. While the original KardiaMobile device has been on the market for awhile, the six-lead iteration reportedly delivers a more detailed look at patients’ hearts, including visibility into certain arrhythmias that act as red flags for CVD.
The six-lead device is consistent in design with the existing KardiaMobile, AliveCor said, but with the addition of an electrode on the bottom of the device for more accurate readings. Users place their thumbs on two electrodes on the device’s surface and place the bottom electrode on their left knee or ankle to measure their heart’s activity. The method, aptly called the Einthoven Triangle, allows cardiologist to view an ECG from six perspectives, or leads.
“I am impressed with the quality and simplicity of six-lead smartphone ECG tracings, which will unquestionably sharpen our ability to diagnose heart rhythm and conduction abnormalities,” Eric Topol, MD, a cardiologist and founder and director of the Scripps Research Translational Institute, said in the release. “It’s a welcome and needed step forward for mobile heart diagnostics.”
The KardiaMobile 6L will be available in June, but it can be pre-ordered at any time on the company’s website.