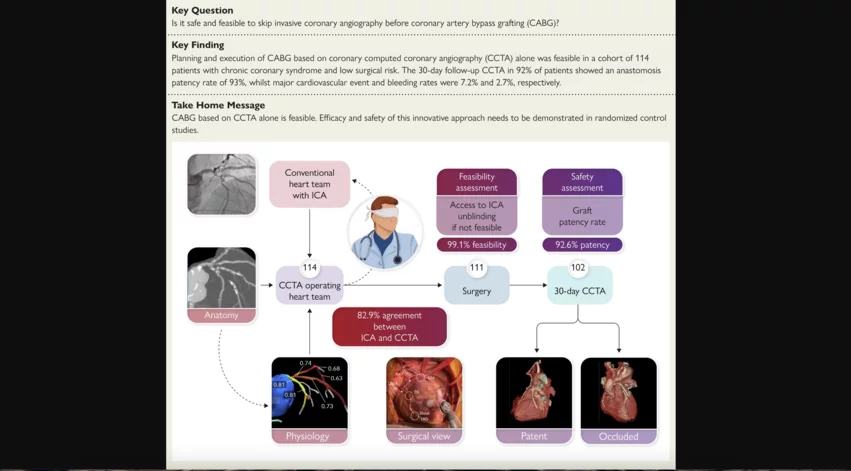
It is both safe and feasible to use coronary computed tomography angiography (CCTA) and fractional flow reserve derived from CCTA (FFRCT) to plan coronary artery bypass grafting (CABG) procedures, according to new research published in European Heart Journal.[1] Importantly, this requires no additional information from invasive coronary angiography (ICA).
Previous studies have already confirmed CCTA and ICA provide comparable insights when heart teams are choosing between CABG and percutaneous coronary intervention (PCI). FFRCT, meanwhile, has gained momentum as a reliable resource when evaluating patients with multivessel disease. The team behind the FAST TRACK CABG study hoped to learn more about how these technologies can guide patient care moving forward.
“The logical next step could have been to plan a trial truly randomizing patients between CCTA and ICA in a blinded fashion,” wrote first author Patrick W. Serruys, an interventional cardiologist with the CORRIB Research Centre for Advanced Imaging and Core Laboratory in Ireland, and colleagues. “However, for ethical reasons, an intermediate step was felt to be mandatory and this is the essence of the FAST TRACK CABG trial: can the surgeon plan his surgery without the visual knowledge of the conventional cineangiography whilst knowing that other surgeons have decided on surgical revascularization?”
Inside the FAST TRACK CABG trial
Serruys et al. explored data from 114 patients who presented with three-vessel disease (3VD) and/or left main (LM) coronary artery disease (CAD). The mean patient age was 65.9 years old, and 87.8% were men. The mean anatomical SYNTAX score was 43.6, and the mean Society of Thoracic Surgery score was 0.81.
ICA and CT were initially performed on each patient so a heart team could make its recommendations. CT images were captured using modern GE Healthcare scanners capable of imaging the heart in a single heartbeat. CCTA images were then sent to an independent laboratory blinded to any ICA findings. The FFRCT technology developed by HeartFlow provided additional insights.
The heart team at that independent laboratory then worked to develop a strategy for each patient’s CABG strategy using only CCTA and FFRCT. They were not able to use ICA results in any way unless there was an issue—in that case, they would be “unblinded” and given access to ICA.
Overall, the independent laboratory was able to plan CABG with just CCTA and FFRCT for 99.1% of patients. For a single patient, the laboratory had to be unblinded to ICA results to make its recommendations.
Concordance and agreement between the original heart team, which had access to ICA findings, and the independent laboratory, which was blinded to ICA, was 82.9%. Concordance and agreement between the independent laboratory and actual treatment was 83.7%.
From a safety perspective, the 30-day major adverse cardiovascular event (MACE) rate for this patient population was 7.2%. The major bleeding event rate, meanwhile, was 2.7%.
“The key finding of this first-in-human study is the 99.1% feasibility, which is driven by the relatively good diagnostic concordance between CCTA and ICA,” the authors wrote. “Overall, the safety and adequacy of surgical revascularization did not appear to be negatively impacted by just relying on CCTA guidance, although it is obviously premature to draw any definitive conclusions, and ultimately, this study will serve as a foundation for large comparative and controlled investigations. Inherently, a first-in-human trial is limited in terms of patient number, and typically, the feasibility and safety of the novel approach is evaluated without a comparative arm since at that stage of the investigation, there are no clinical data available to justify any rational sample size or to guarantee the safety of a large, powered randomization study.”
GE Healthcare and HeartFlow both helped fund FAST TRACK CABG through grants, but they did not help design the study, gather data or write the text.
Additional research is still needed to confirm these findings. Click here to read the full study in European Heart Journal, a publication of the European Society of Cardiology.