FDA OKs 1st implantable continuous glucose monitor
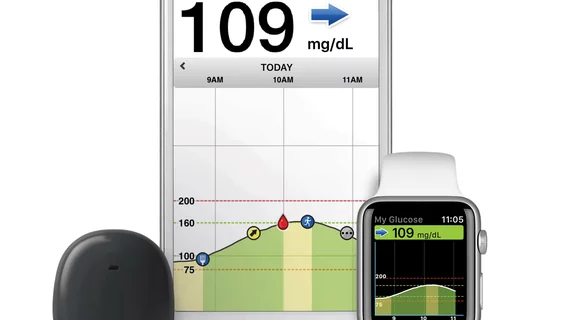
The FDA announced on June 21 it has approved the Eversense Continuous Glucose Monitoring (CGM) system, the first fully implantable product of its kind. The device can be worn for up to 90 days, whereas most CGMs are replaced every week or two.
“The FDA is committed to advancing novel products that leverage digital technology to improve patient care,” FDA Commissioner Scott Gottlieb, MD, said in a statement. “These technologies allow patients to gain better control over their health. This approval of a more seamless digital system that gives patients the ability to effectively manage a chronic disease like diabetes is a vivid illustration of the potential for these mobile platforms.”
The device is manufactured by Sensonics Holdings and is approved for patients 18 and older with type 1 or type 2 diabetes. It is implanted just under the skin by a physician and uses light-based technology to measure glucose levels, then alerts users via a mobile app if readings are too high or too low.
The FDA based its approval on a 125-patient clinical study that showed a serious adverse event rate of less than 1 percent, along with comparable monitoring capabilities to a laboratory-based glucose analyzer.
"We're very pleased to receive this FDA approval that allows us to make Eversense available in the United States, as it is in many European markets,” Tim Goodnow, president and CEO of Senseonics, said in a press release. “With the parallel trends of wearable personal devices and medical implantables for people to manage their health, this product exemplifies the natural evolution for diabetes devices, and Senseonics is excited to help lead the way. More importantly, we believe the unique features Eversense offers will help open up CGM to millions of people with diabetes who, up to this point, have been hesitant to try CGM despite the clear health benefits it provides."
According to the FDA, the safety of this device will be further evaluated in a post-approval study.