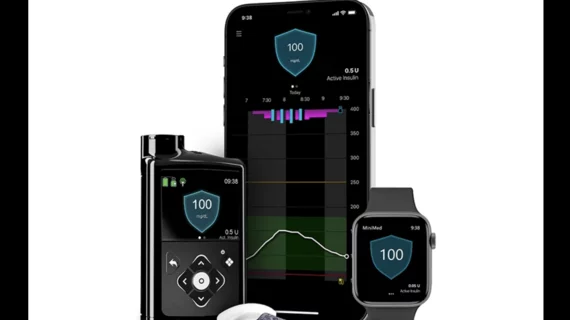
Medtronic’s new insulin pump with meal detection technology has been approved by the U.S. Food and Drug Administration (FDA). The MiniMed 780G system is approved for patients with type 1 diabetes seven years old and older. Patients currently using the MiniMed 770G system can upgrade at no additional cost.
According to Medtronic, the MiniMed 780G features the lowest target glucose setting on the market—100 mg/dL—and includes an infusion set that allows it to be worn up to seven days longer than normal. When combined with Medtronic’s Guardian 4 sensor, the device does not require fingerstick tests from the user to obtain a reading.
One key feature available on the new pump is meal detection technology, which can deliver insulin in those moments when the user loses track of time or underestimates how a meal may impact their health.
“Mealtimes prove to be one of the biggest challenges for people living with type 1 diabetes and now for the first time, the MiniMed 780G system addresses this unmet need with automatic, real-time insulin corrections,” Que Dallara, executive vice president and president of Medtronic’s diabetes division, said in a prepared statement. “A lot can happen to blood sugars in the span of an hour or even just a few minutes, so we've designed our system for real life—the algorithm adapts to the user and helps compensate for everyday challenges that are quite common around mealtimes. We built in features informed by extensive customer feedback and we're excited to deliver a system with ease of use at the forefront.”
The FDA’s approval was based in part on clinical data first shared with the public in June 2020. Researchers found that the MiniMed 780G was linked to a time in rage of 78.8%.
The MiniMed 780G system has been available in Europe since 2020. It is now expected to start shipping to customers by the end of summer.