Cordis has agreed to acquire MedAlliance, a Switzerland-based healthcare technology company focused on drug-eluting balloons, for an initial investment of $35 million and an upfront closing payment of $200 million. By 2029, if the companies reach all targeted regulatory and commercial milestones, the acquisition could cost Cordis up to $1.135 billion in all.
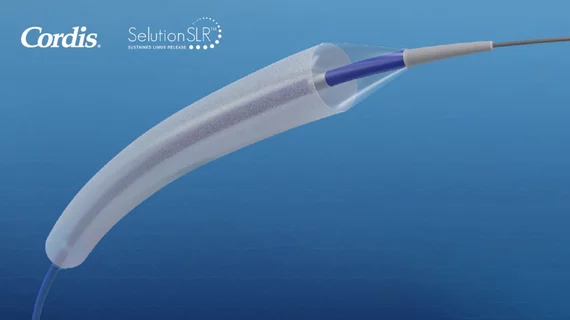
Cordis pursued this deal due to its interest in MedAlliance’s family of Selution SLR drug-eluting balloons, which were designed to include “MicroReservoirs” made of biodegradable polymer and sirolimus. This allows sirolimus to be released at a controlled and sustained pace for up to 90 days, a technique that has been proven to benefit patients undergoing both coronary and peripheral procedures.
The devices are already available in Europe for the treatment of peripheral artery disease and coronary artery disease. They have also been awarded four different breakthrough designations from the U.S. Food and Drug Administration (FDA), and MedAlliance has started enrolling patients for multiple clinical studies in the United States aimed at securing full approval from the FDA.
“The acquisition of MedAlliance illustrates our vision to maximize patient impact by pairing highly innovative growth drivers with Cordis’ trusted brand and extensive global commercial capabilities,” Duke Rohlen, executive chairman of Cordis, said in a prepared statement.
“Nearly 20 years ago, Cordis introduced Cypher, the first drug-eluting stent, transforming cardiovascular treatment for patients around the world,” added Shar Matin, Cordis CEO. “Today, we are furthering that legacy of innovation and market disruption with MedAlliance and the first MicroReservoir sirolimus drug-eluting balloon, Selution SLR.”
“We are very fortunate to find a partner like Cordis, with its strong history of innovation,” Jeffrey B. Jump, chairman and CEO of MedAlliance, said in another statement. “The company that introduced sirolimus drug eluting stents in 1999 will be introducing the Selution SLR sustained limus release sirolimus drug eluting balloons, avoiding permanent metal implants and providing patients around the world with stentless percutaneous coronary intervention (PCI).”