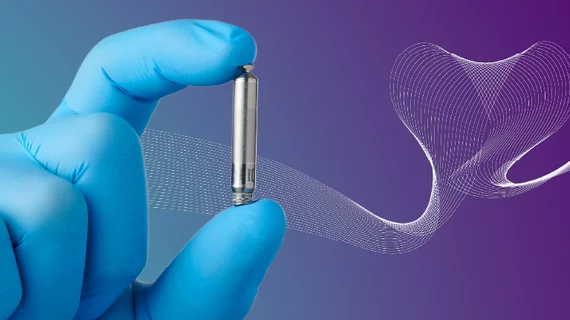
Abbott’s single-chamber leadless pacemaker gains FDA approval
The U.S. Food and Drug Administration (FDA) has approved Abbott’s Aveir single-chamber (VR) leadless pacemaker as a treatment option for patients with slow heart rhythms. It is the second VR leadless pacemaker to gain approval in the United States, joining Medtronic’s Micra device.
According to Abbott, clinicians will be able to use a new mapping feature to ensure the best possible placement before implanting the device. The company also said that it was designed to have an especially long battery life, and it can be retrieved when necessary if changes need to be made to the patient’s treatment strategy.
“The Aveir VR leadless pacemaker was designed to make the implantation and retrieval processes as seamless as possible for physicians and provide improvements over existing options,” Randel Woodgrift, Abbott’s senior vice president of cardiac rhythm management, said in a statement. “Our goal is to continue to build on the success of Aveir to provide more first-of-their-kind products in the future, revolutionizing how abnormal heart rhythms are treated.”
“The Aveir leadless pacemaker brings unique innovations we've been seeking, such as the ability to ensure electrical performance before we commit to placement,” added Rahul Doshi, MD, director of electrophysiology at Honor Health. “Abbott's leadless pacemaker addresses the need for a single-chamber device that accommodates any therapy path for a patient through Aveir's retrieval capability and extended battery longevity.”
Doshi was one of several co-authors who evaluated the Aveir VR leadless pacemaker for Abbott. Findings from the team’s LEADLESS II-Phase 2 trial were published in JACC: Clinical Electrophysiology in January 2022.[1]
“This single-chamber leadless pacemaker is designed to provide an expandable platform to later support a fully leadless dual-chamber pacing system once approved,” they wrote at the time.
Related Heart Rhythm Content:
First implant of dual-chamber leadless pacemaker completed in Abbott’s Aveir DR i2i pivotal trial
VIDEO: 4 predictions on key cardiac technologies for the coming years
DOACs may reduce the risk of dementia among AFib patients by 50%
Myocarditis, arrhythmias and more: An updated look at what cardiologists know about long COVID-19
Reference: