FDA announces new insulin recall due to potential labeling issue
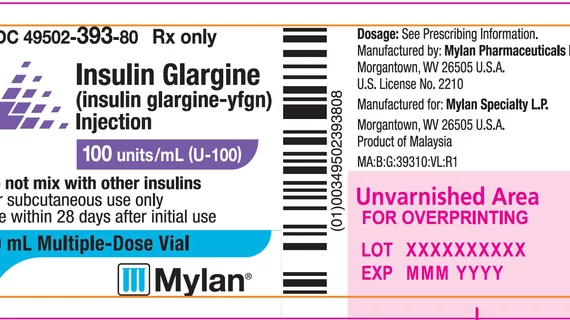
Mylan Pharmaceuticals, a Viatris Company, is recalling one batch of its Insulin Glargine injections due to the risk of labels missing from some vials.
The batch, distributed from December 2021 to March 2022, included 10-mL vials of the company’s unbranded insulin glargine-yfgn, which is designed to control glycemic control in adult patients diagnosed with type 1 diabetes or type 2 diabetes. It can also be prescribed to pediatric patients diagnosed with type 1 diabetes.
“For patients receiving treatment with more than one type of insulin (e.g., both short and long-acting insulin), a missing label on Insulin Glargine vials could lead to a mix-up of products/strengths, which may result in less optimal glycemic control (either high or low blood sugar) which could result in serious complications,” according to an advisory on the U.S. Food and Drug Administration (FDA) website. “To date, no adverse events related to this recall have been received for this product.”
Retailers are asked to examine their inventory and discontinue the distribution of any included products. If consumers discover they have been sold an unlabeled product, they can return it.
“Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product,” according to the advisory.
Viatris first announced the U.S. launch of its unbranded insulin glargine-yfgn injections back in November 2021. They were made available in both vials and prefilled pens. Additional information is available here.
Related Diabetes and Pharmaceutical Content:
VIDEO: Use of mRNA drug to lower lipoprotein(a) by up to 98%
AI model able to ID early signs of type 2 diabetes on imaging results
4 new medications and technologies that could transform cardiology in 2022
Adults with type 2 diabetes are not taking care of their hearts
Verapamil shows potential as a long-term oral treatment for type 1 diabetes
Obesity more common among patients with type 1 diabetes than ever
Adults with COVID-19 and type 1 diabetes face a heightened risk of hospitalization
Cholesterol medications, flu shots and heart failure: Day 2 at ACC.22
Salt restrictions, PCI breakthroughs and a social media primer for cardiologists: Day 1 at ACC.22