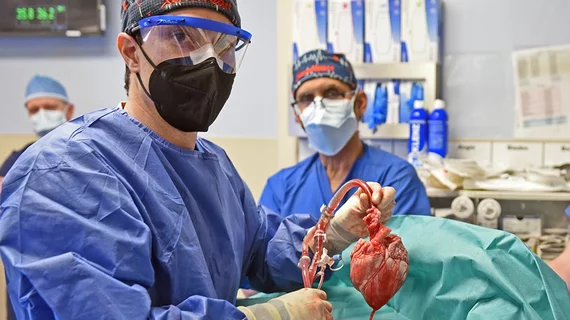
FDA may allow clinical trials of pig organ transplants into human patients
The U.S. Food and Drug Administration (FDA) may soon allow researchers to perform clinical trials focused on the transplantation of pig organs into human patients. That significant update from the agency comes courtesy of a new report from The Wall Street Journal.
The report, published June 30, is based on an anonymous source close to the situation. The Wall Street Journal’s source also said these trials would likely be approved on a case-by-case basis. The exact timing of this update is unknown.
The most successful case to date of a pig organ being transplanted into a human occurred back in January, when specialists at the University of Maryland Medical Center (UMMC) in Baltimore transplanted a modified pig heart into 57-year-old David Bennett. The FDA approved the heart transplant transplant through an emergency authorization typically reserved for experimental procedures seen as a patient’s last chance at survival. Bennett did die of heart failure two months later, but UMMC specialists had been “incredibly encouraged” by his progress before his condition worsened.
“We consider this to be an important learning experience,” Muhammad M. Mohiuddin, MBBS, program director of UMMC’s cardiac xenotransplantation program, said in a Jan. 22 statement. “Knowing what we know now, we will alter some of our practices and techniques in the future.”
Another significant pig-to-human transplant from this year occurred at the University of Alabama in Birmingham, where gene-edited pig kidneys were transplanted into a brain-dead human.
Click the link below for the full story: