TricValve device proven safe and effective after 6 months for patients with severe symptomatic TR
The TricValve Transcatheter Bicaval Valves System is associated with a high procedural success rate and strong outcomes after six months, according to new research published in JACC: Cardiovascular Interventions.[1]
“Heterotopic bicaval stenting, or caval valve implantation, has emerged as a possible transcatheter strategy for indirectly treating the systemic effects of severe tricuspid regurgitation (TR),” wrote first author Rodrigo Estévez-Loureiro, PhD, an interventional cardiologist with University Hospital Alvaro Cunqueiro in Spain, and colleagues. “This approach carries the inherent advantages of a streamlined fluoroscopic procedural workflow using familiar concepts akin to transcatheter aortic valve replacement.”
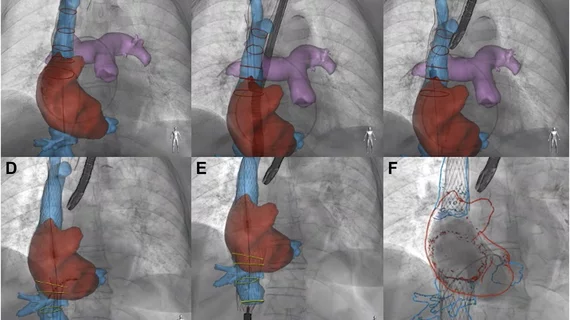
A bit of context about the TricValve Transcatheter Bicaval Valves System
The TricValve system features two biological valves designed to be implanted via femoral vein access into the patient’s superior vena cava and inferior vena cava. This allows a therapy without impacting the patient's native tricuspid valve. It is available in multiple sizes, allowing cardiologists to choose the best option for each individual patient.
The system was developed by P+F Products + Features GmbH, a healthcare technology company based out of Vienna, Austria. The solution was granted the U.S. Food and Drug Administration’s Breakthrough Device designation in 2020, but it has not gained full approval.
In February 2020, interventional cardiologists at Cleveland Clinic successfully completed the first implantation of the TricValve device in North America. The patient, 82, presented with severe symptomatic TR and right heart failure.
Key findings from the TRICUS EURO trial
The TRICUS EURO trial included 35 patients with a mean age of 76 years old. All patients were treated at one of 12 facilities in Spain or Austria, and 83% of patients were women. Each patient presented with symptomatic severe TR, and echocardiography results showed signs of significant backflow of the inferior vena cava and/or superior vena cava.
The procedural success rate among these patients was 94%. There were no deaths, and no patients had to be converted to surgery. After six months of follow-up, the authors added, there was a “significant increase” in quality of life (QOL) as measured by Kansas City Cardiomyopathy Questionnaire scores and New York Heart Association functional class improvement.
The authors also reviewed CT results after three months and saw no signs of perforation or structural damage to the valve stents. Two cases of device thrombosis were observed, but no clinical events were reported for either patient; they were both treated with anticoagulation therapy.
“The current target population of transcatheter therapies for severe TR is typically old and frail, with significant comorbidities and decreased QOL,” the authors wrote. “In such individuals, the immediate goals of care are to improve QOL and reduce hospitalization rates. TRICUS EURO demonstrated a significant, rapid QOL improvement at 30 days that was sustained at six months.”
The group did note that additional research is still required, including more evaluations of how this treatment option compares to standard medical therapy. Also, there were considerable increases in patient NT-proBNP levels after three months.
Related Structural Heart Disease Content:
Interventional cardiologists complete first heart procedure of its kind in North America
VIDEO: Advances in transcatheter tricuspid and mitral valve technology
VIDEO: Transcatheter tricuspid devices likely to gain FDA clearance before new mitral advances
Reference: