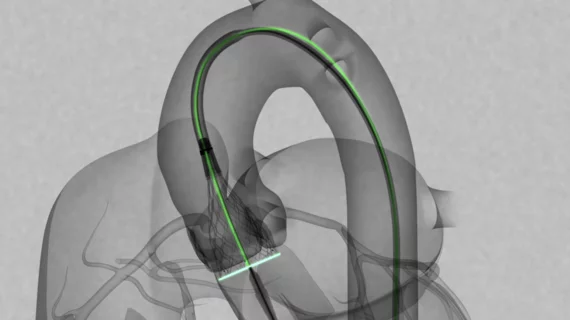
Medtronic launches Evolut FX TAVR system for severe aortic stenosis
Medtronic has announced the full commercial launch of the newest addition to its Evolut transcatheter aortic valve replacement (TAVR) platform, the Evolut FX TAVR system. The self-expanding, supra-annular TAVR device gained FDA approval back in August 2021 as a treatment option for symptomatic severe aortic stenosis. It is indicated for both high- and low-risk patients.
The Evolut FX device includes certain updates not seen in previous Evolut systems, including gold markers that assist with commissure alignment, an updated catheter tip designed to help with insertion and a more flexible delivery system. It includes four valve sizes.
“This represents a milestone for our structural heart business, as we look to set new expectations for TAVR delivery systems and optimize outcomes for patients,” Jeffrey Popma, MD, vice president and chief medical officer for Medtronic’s structural heart and aortic business, said in a prepared statement. “These innovations will equip physicians with improved implant predictability, ultimately improving the overall reliability of the procedure.”
Medtronic’s news came as specialists and vendors in the structural heart disease and interventional cardiology spaces were gathering for Transcatheter Cardiovascular Therapeutics (TCT) 2022 annual meeting in Boston. Multiple presentations at the four-day conference focused on Medtronic devices and data, as the company detailed in its announcement.
Additional TAVR content is available here.