FDA announces another recall for troubled heart device after multiple hospitalizations
The U.S. Food and Drug Administration (FDA) has shared the details of another recall associated with Medtronic’s HeartWare Ventricular Assist Device (HVAD) system.
This most recent recall is focused on the HVAD system’s driveline boot cover, which “can become stiff over time” and “be difficult to disconnect … from the controller.” Medtronic received a total of 33 complaints from January 2017 to September 2022 about the issue. In four of those instances, the patient had to be hospitalized so the driveline cover could be serviced as needed. No critical health issues or deaths were reported.
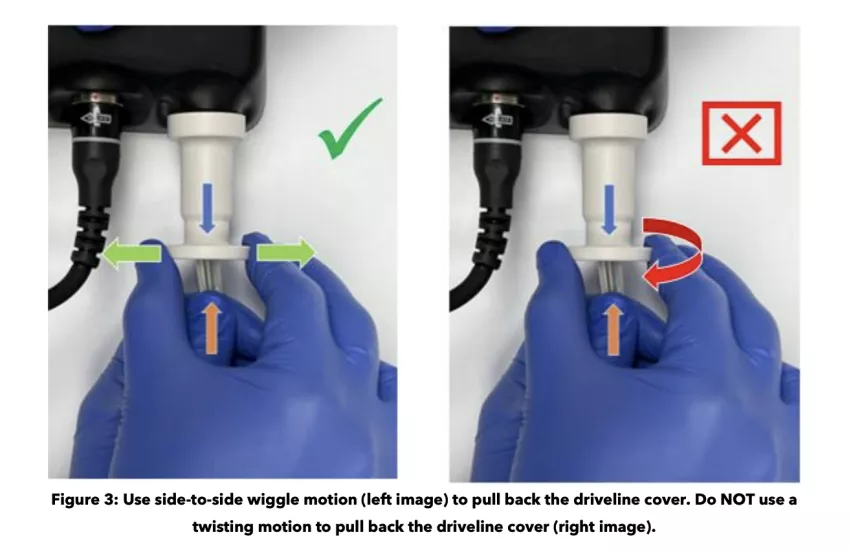
Medtronic has shared instructions with customers for evaluating the driveline cover of any HVAD systems in their possession. A “side-to-side wiggle motion” is recommended, and pulling the cover too hard “may damage the driveline connector or dislodge the driveline connection.”
“Medtronic advises that hardened boot covers be reported to them so the need to perform a field service procedure to remove the cover can be discussed,” the FDA reported on its website.
The HVAD system has been a consistent source of problems for Medtronic for quite some time. The FDA’s website lists more than a dozen recalls linked to the device, and Medtronic actually stopped selling and distributing it back in June 2021 due to these issues.
“The FDA has monitored the performance of the Medtronic HVAD System since it was approved in 2012, including monitoring neurological adverse event rates,” the FDA said at the time. “Although Medtronic has stopped the sale and distribution of new HVAD Systems, the FDA will continue to monitor the safety and effectiveness of the HVAD systems that remain implanted.”
Read Medtronic’s full medical device communication about the potential driveline problems here. The document, dated Nov. 29, includes multiple photos and thorough recommendations. It also includes information for reporting any adverse events or quality problems with the device.