Interventional cardiology specialists raise $20M to continue IVL research
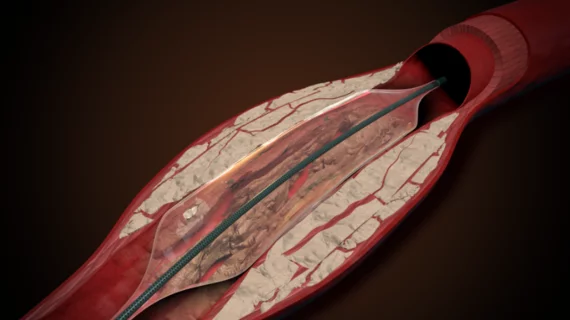
AVS, a Michigan-based medical device company focused on interventional cardiology, has raised $20 million in Series B financing. The funding is expected to help AVS ramp up work on its Pulse Pulsatile-Intravascular Lithotripsy (P-IVL) system, a new device designed to treat peripheral artery disease (PAD) by breaking down calcium deposits and expanding calcified arteries.
The POWER PAD 1 clinical trial, designed to examine the safety and effectiveness of the Pulse IVL system, is now underway. This first-in-human evaluation will include data from a cohort of 20 patients, and AVS has said that initial findings from the trial are positive.
“Our early trial results showed that we can successfully treat patients with multiple lesions using a single device,” Jon George, MD, an interventional cardiologist with the University of Pennsylvania health system and medical advisor for AVS, said in a prepared statement. “We saw patients report a reduction in leg pain, increase in blood flow to the leg, and improvement in their ability to walk in our initial study. This is a patient population that needs easier access to advanced therapies and this platform has the potential to provide that access.”
“AVS is one step closer to offering a new treatment solution for patients with severely calcified peripheral arterial disease and progressing toward preclinical studies for coronary cases,” added Mark Toland, executive chairman and CEO of AVS. “Patients in this disease state too often face the prospect of limb amputation due to a lack of treatment options. We see a significant opportunity to address that need and advance the intravascular lithotripsy space through minimally invasive technology in both peripheral and coronary therapy.”
Toland also serves as the managing director of BioStar Capital, the investment firm that led this funding round. BioStar Capital previously led AVS’s Series A funding round.
“We are excited to partner with AVS as it looks to future clinical trials and development,” Louis Cannon, MD, founder and senior managing partner of BioStar Capital, said in the same statement.
The Pulse P-IVL system is still in development and it has not yet been cleared for commercial use in any country.