Day 3 at ACC.23 features late-breaking studies on pulsed-field ablation, other new technologies
The American College of Cardiology’s annual meeting, ACC.23 Together with the World Congress of Cardiology, concluded just as it started: with a healthy serving of late-breaking research and fascinating studies.
Catch up on the busy day below:
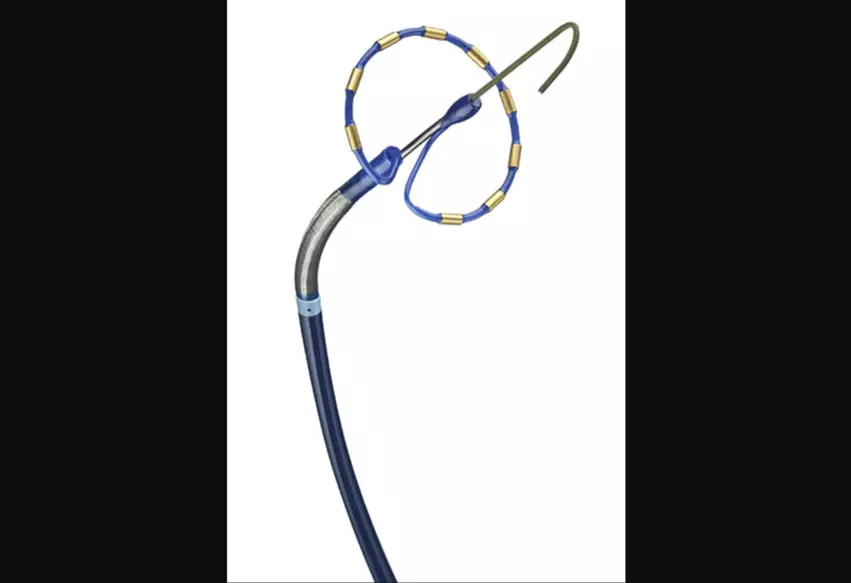
PULSED AF trial shows improved safety with pulsed field ablation
The day kicked off with one of the weekend’s most highly anticipated late-breakers. Atul Verma, MD, head of cardiology at McGill University Health Centre in Montreal, presented updated data on Medtronic’s pulsed-field ablation technology in patients presenting with atrial fibrillation (AFib). Pulsed-field ablation is different from both cryoablation and radiofrequency ablation techniques—it uses electrical fields as opposed to heat or extreme cold to reach the targeted cardiac cells.
The PULSED AF trial focused on 300 procedures performed in the United States, Canada, Australia, Austria, Belgium, France, Japan, the Netherlands and Spain. All patients were treated with Medtronic’s PulseSelect Pulsed Field Ablation System, which has not yet been fully approved by the U.S. Food and Drug Administration. Each operator’s first pulsed-field ablation procedure was not included in the analysis, giving them an opportunity to gain experience with the new-look technology.
While half of the study’s patients presented with paroxysmal AFib, the other half presented with persistent AFib. They were all followed for one year. Overall, Verma reported, pulsed-field ablation with the Medtronic device was found to be safe, effective and associated with “clinically meaningful improvements” in quality of life as a treatment option for both paroxysmal and persistent AFib. Acute isolation was seen in 100% of all pulmonary patients.
“Today's findings demonstrate a critical achievement in the decade-long commitment we have made to researching PFA and underscores our confidence in the PulseSelect System,” Rebecca Seidel, president of Medtronic’s Cardiac Ablation Solutions business, said in a prepared statement.
The full study is now available in Circulation. Read the findings here.
New cardiac technologies show potential in ACC late-breaking studies
Partho P. Sengupta, MD, a cardiology professor with Rutgers Robert Wood Johnson Medical School, presented late-breaking data on a new wrist-worn device designed to use artificial intelligence to estimate cardiac troponin-l (cTnl) levels and guide treatment decisions—all without drawing the patient’s blood.
The analysis focused on data from 239 patients who presented with myocardial infarction symptoms. All patients had their blood drawn, underwent an electrocardiogram and either ran echocardiogram or coronary angiogram.
Overall, Sengupta’s team found that, yes, this new transdermal infrared spectrophotometric sensor can feasibly make AI-based cTnl assessments by being attached to a patient’s wrist. In fact, its accuracy was measured at 90%, a promising sign for this technology going forward.
“Transdermal infrared-based techniques open up a tremendous potential for bloodless biomarker assessment,” Sengupta noted in an ACC statement. “We have started with troponin, but the journey is going to continue because it is possible to use this technology for other biomarkers. This is just the start.”
John P. Boehmer, MD, a professor of medicine and surgery with Penn State University, presented another late-breaking clinical trial focused on the future. Boehmer’s team investigated the use of a wearable sensor capable of evaluating the amount of fluid in a user’s lungs.
The ZOLL Heart Failure Management System (HFMS), developed by ZOLL, was associated with a 38% reduction in hospital readmissions among heart failure patients after an initial heart failure hospitalization.
“ZOLL HFMS has demonstrated the ability to reduce hospital readmissions by providing remote monitoring of pulmonary fluid level changes, giving clinicians a new capability in their toolbox to care for patients with heart failure and improve outcomes,” Jason T. Whiting, Zoll’s president of Cardiac Management Solutions, said in a statement.
After his presentation, Boehmer noted that, in his experience, patients always appreciate seeing their data when wearing a monitoring device. During this trial, patients had full access to their pulmonary fluid levels, a detail highlighted by the ACC.22/WCC panelists.
A new intervention for cardiologists
Neha J. Pagidipati, MD, associate professor of medicine in cardiology with the Duke University School of Medicine, presented late-breaking data focused on improving care for patients presenting with type 2 diabetes and cardiovascular disease.
The COORDINATE-Diabetes study included cardiologists from 43 different clinics. Each clinic either received straightforward, simple instructions or underwent a six-part intervention designed in improving the use of statins, angiotensin converting enzyme inhibitors/angiotensin receptor blockers and sodium glucose cotransporter-2 inhibitors. Overall, after approximately one year, 14.5% of patients in the “simple education” arm were prescribed all three medication classes and 38% of patients in the intervention arm were prescribed all three medication classes.
Pagidipati highlighted just how valuable it is to get these therapies in the hands of the patients who need them.
Stay tuned for much more coverage from ACC.23/WCC.