FDA announces recall of cryoablation catheters after multiple injuries, including 4 deaths
The U.S. Food and Drug Administration (FDA) has announced that Boston Scientific is recalling the catheters associated with its POLARx Cryoablation System due to a heightened risk of esophageal injury. The issue has been linked to seven patient injuries and four deaths.
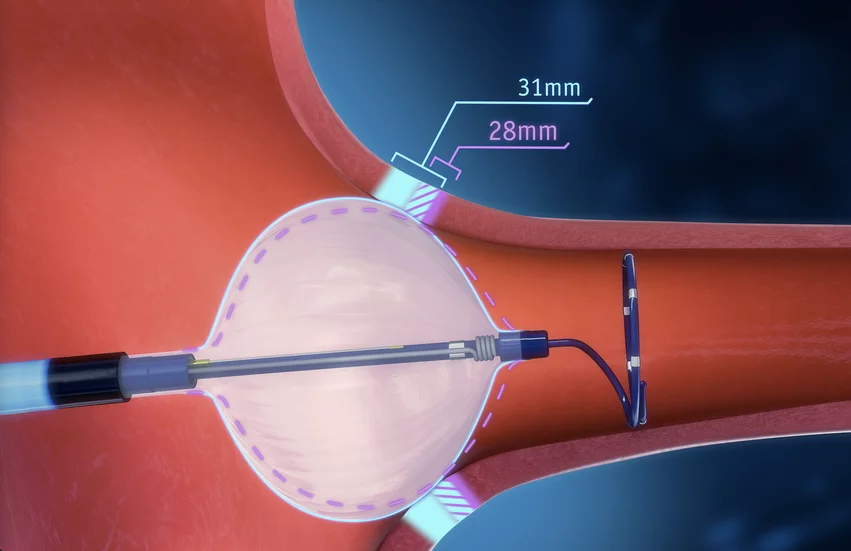
The POLARx Cryoablation System is designed to treat recurrent, symptomatic atrial fibrillation that does not respond to treatment from medical therapy alone. It gained FDA approval back in August 2023.
The FDA has ruled that this is a Class I recall, which means it is associated with the highest possible risk level. However, this recall does not involve removing the devices from the market. Instead, Boston Scientific has updated the instructions for use and is urging customers to follow these updated instructions moving forward.
The recall includes both the POLARx and POLARx FIT cryoablation catheters.
The updated instructions for use from Boston Scientific highlight the importance of “verifying balloon position, esophageal monitoring and phrenic nerve monitoring.” In addition, the company has warned that ablations should not be immediately repeated in the same location, and ablation should be stopped if the balloon temperature decreases to -65° C.
“The updates emphasize the risk of atrio-esophageal fistula and practices that may minimize risk, based on the observation that location, frequency and intensity of cryoablation applications may be contributing factors to this complication,” according to the FDA advisory. “The use of affected product may cause serious adverse health consequences, including atrio-esophageal fistula leading to air bubbles blocking blood vessels in the brain (cerebral air embolism), stomach and intestinal (gastrointestinal) bleeding, a system-wide infection (septic shock), and death.”