Abbott’s Amulet LAAO device linked to long-term safety, effectiveness
The Amplatzer Amulet Left Atrial Appendage Occluder (LAAO) from Abbott is safe and effective a full five years after treatment, according to new data published in the Journal of the American College of Cardiology.[1]
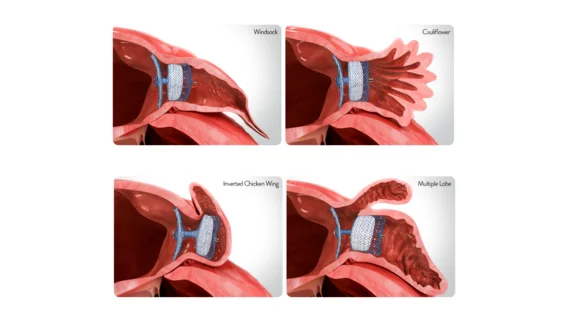
The study’s authors reviewed data from more than 1,800 high-risk patients with nonvalvular atrial fibrillation (AFib). Patients were randomized to undergo LAAO with either the Amulet, which seals the left atrial appendage at the ostium and the neck, or Boston Scientific’s Watchman 2.5 single-closure device. All procedures were originally performed from September 2016 to March 2019 as part of the Amulet IDE trial funded by Abbott.
Overall, researchers found that the Amulet and Watchman were associated with comparable rates of ischemic stroke or systemic embolism, major bleeding events, all-cause death and cardiovascular death after five years, highlighting the safety and effectiveness of both devices.
“The long-term five-year outcomes presented in this analysis offer valuable insights into the durability of LAAO and help guide clinical decision making for stroke reduction in patients with nonvalvular AFib,” wrote first author Dhanunjaya Lakkireddy, MD, a cardiologist with the Kansas City Heart Rhythm Institute, and colleagues.
Lakkireddy et al. did identify certain differences between the two LAAO treatment options. For example, 78.9% of Amulet patients were free of oral anticoagulation (OAC) immediately after discharge compared to just 4.2% of Watchman patients. By the end of the study, these rates were much closer; 94% of Amulet patients and 90.9% of Watchman patients were free of OAC after five years.
Another difference between the two devices was the fact that Watchman patients were associated with more fatal or disabling strokes (39) than Amulet patients (22). This could be because the Watchman was linked to an increased risk of device-related thrombus (DRT) or peridevice leaks (PDL), researchers wrote.
The Amulet and Watchman devices have both been approved by the U.S. Food and Drug Administration for treating nonvalvular AFib in patients at a heightened stroke risk. The Amulet gained FDA approval in 2021, and the Watchman gained approval back in 2015.
Click here to read the team’s full analysis.
Reassuring 5-year findings
A separate editorial, also published in the Journal of the American College of Cardiology, provided additional insights into this latest update on the safety and effectiveness of LAAO.
“The results from the Amulet IDE trial reassure us that the available devices in the United States both offer consistent and excellent efficacy with acceptable safety,” wrote corresponding author James. V. Freeman, MD, MPH, MS, a cardiologist with Yale School of Medicine, and colleagues. “Additionally, these data suggest that the next most important frontier for improving the net clinical benefit of percutaneous LAAO is determining the optimal postimplantation antithrombotic regimen.”
Freeman et al. also examined the difference in fatal/disabling strokes between these two devices, highlighting that the performances would likely be much closer if a more recent version of the Watchman device had been used for this analysis.
“Although the first-generation Amulet remains the only commercially available Amulet device, second- and third-generation Watchman devices have been commercially released, with multiple design changes that have been shown to reduce the risk for pericardial effusion, major bleeding, PDL and likely DRT,” they wrote.
Click here to read their full commentary.