FDA approves Boston Scientific’s new cryoablation system for AFib
The U.S. Food and Drug Administration (FDA) has approved Boston Scientific’s POLARx Cryoablation System for the treatment of patients with atrial fibrillation (AFib).
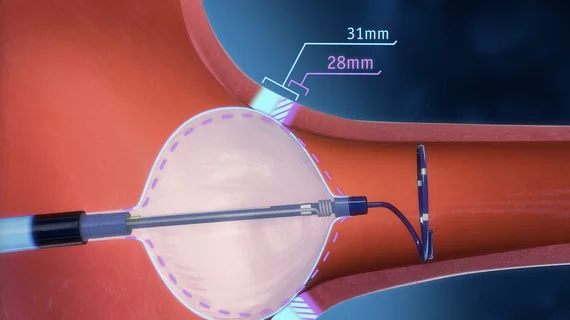
The system includes the expandable POLARx FIT cryoablation balloon catheter, which is compatible with both a 28-mm balloon and a 31-mm balloon. This was designed to save operators time and give them more freedom to adjust the device during procedures.
The vendor said it was also designed with an ultra-maneuverable catheter sheath so it is easier to navigate, intuitive console and improvements to speed workflow in the EP lab.
“The U.S. approval of the POLARx Cryoablation System, which has been used in more than 25,000 patients worldwide to date, marks an exciting advancement for the treatment of AFib and a new era of cryoablation capabilities,” Nick Spadea-Anello, Boston Scientific’s president of electrophysiology, said in a prepared statement. “By prioritizing procedural flexibility and individualized care, this offering transforms a key therapy in the electrophysiology space, addresses the unmet needs of physicians and affirms our commitment to making meaningful innovations to established technologies.”
The FDA’s decision to approve the new-look catheter was in part due to results from the FROZEN-AF investigational device exemption trial, which included data from 385 patients who presented with paroxysmal AFib. One year after treatment, 96% of patients were free of procedure- or device-related events, and the rate of freedom from documented atrial arrhythmias was nearly 80%.
Wilber Su, MD, director of electrophysiology at Banner University, is familiar with the newly approved catheter. In the same statement, he highlighted its ability to “better tailor care for individual patients without sacrificing safety or efficiency.”
“As we saw in clinical evaluation, the combination of maneuverability and variable balloon sizes makes this system particularly useful in addressing longstanding challenges with varying cardiac anatomies and brings to the table occlusion capabilities physicians aren't used to seeing with traditional systems,” Su added.
The POLARx Cryoablation System received European CE mark approval in February 2020. It has also been approved for regular use in Japan, Canada and other markets.