Abbott gains CE mark approval for Volt PFA system
Abbott has received CE mark approval in Europe for its new Volt Pulsed Field Ablation (PFA) System to be used for the treatment of patients presenting with atrial fibrillation (AFib). The approval, which Abbott said arrived earlier than expected, came after regulators reviewed data from a global clinical trial the company launched in January 2024.
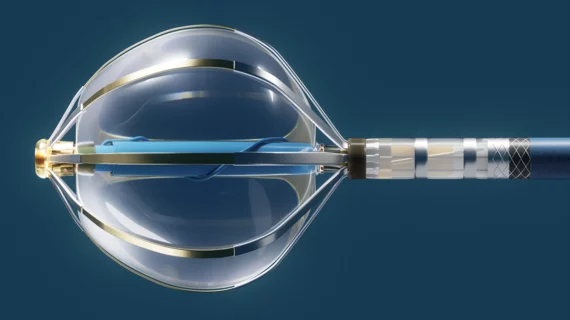
According to Abbott, Volt has been paired with the company’s EnSite X EP heart mapping technology to make PFA procedures more accurate and limit the number of necessary ablations. The company also noted that the proprietary balloon-in-basket design of the Volt’s catheter helps clinicians target tissue with an increased accuracy.
“While PFA is a relatively new therapy option, we've incorporated lessons learned from first-generation devices and designed the Volt system to simplify PFA procedures while making them more efficient,” Christopher Piorkowski, MD, chief medical officer of Abbott's electrophysiology business, said in a statement. “Clinical data has also shown that the Volt catheter's cutting-edge design helps physicians achieve pulmonary vein isolation in fewer ablation attempts and less therapy applications for improved patient outcomes.”
“The launch of Abbott's Volt PFA system marks a major milestone in the evolution of electrophysiology across Europe and signals we're moving beyond early therapy approaches to new systems that incorporate key physician feedback and clinical insights to optimize PFA therapy,” added Volt user Helmut Pürerfellner, MD, head of the department of electrophysiology at Ordensklinikum Hospital in Linz, Austria. “PFA is significantly changing our approach to treating patients and it's exciting to see the Volt PFA System build on the therapy's potential and bring new benefits to clinical teams so we can improve the lives of more patients battling conditions like AFib.”
The Volt PFA System has not yet been approved by the U.S. Food and Drug Association to be commercially used in the United States.
PFA offerings already approved by the FDA include Medtronic’s PulseSelect and Affera systems, Boston Scientific’s Farapulse system and Johnson & Johnson MedTech’s Varipulse Platform.
EP experts say PFA offers increased safety over traditional ablation catheters by more precisely treating target tissue, and preventing major adverse events, such as atrio-esophageal fistula and phrenic nerve injury.