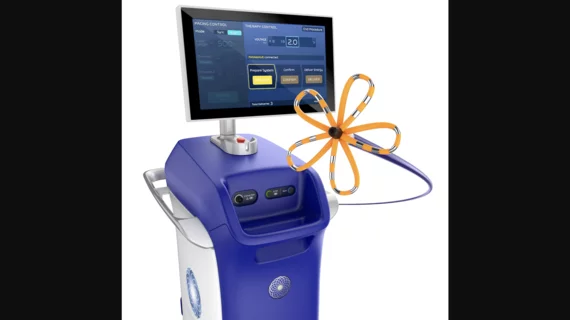
FDA approves Boston Scientific’s pulsed field ablation system for AFib
Boston Scientific’s Farapulse Pulsed Field Ablation (PFA) System has been approved by the U.S. Food and Drug Administration (FDA) to treat patients with symptomatic, paroxysmal atrial fibrillation (AFib), making it just the second PFA offering cleared for commercial use in the United States. Medtronic’s PulseSelect PFA System gained FDA approval in December and was the very first device of its kind to gain FDA clearance.
The Farapulse PFA system has been gaining momentum in recent years, earning the FDA’s breakthrough device designation back in 2019 and then gaining CE mark approval in 2021. It treats AFib with tissue-selective, non-thermal electric fields designed to ablate the operator’s target heart tissue without doing damage to the surrounding area. The technology uses electroporation, where the pulsed electrical fields create openings in the cell walls that lead to cell death, rather the current standard of care using heat or cold. In trials, PFA technology was found to be safer than radiofrequency or cryoablation.
“The approval of the Farapulse PFA System marks an important milestone for the millions of people living with paroxysmal AFib and is an incredible opportunity to bring the first PFA system designed and built solely for this type of ablation therapy to physicians in the U.S.,” Nick Spadea-Anello, Boston Scientific’s president electrophysiology, said in a prepared statement. “A high bar has been set by performance of the system in clinical and commercial settings—where more than 40,000 patients have been treated to date—and we look forward to continuing to lead the way with this differentiated technology in the growing PFA space.”
The FDA’s final decision was based largely on results from the ADVENT clinical trial, which compared treatment with the Farapulse system with radiofrequency and cryoballoon ablation. The Boston Scientific offering was found to be noninferior to these other ablation options, and researchers reported that the technology was relatively easy for first-time operators to use.
“Within the ADVENT clinical trial, the Farapulse PFA System was shown to be a safe, effective and efficient option for treating paroxysmal AF, and extensive global real-world use has mirrored that profile,” ADVENT principal investigator Vivek Reddy, MD, director of electrophysiology with Mount Sinai Fuster Heart Hospital, said in the same Boston Scientific statement. “Tissue preferentiality and long-term efficacy, combined with markedly shorter procedure times and learning curves, position the Farapulse PFA System with strong potential to become a practice-changing technology for both U.S. physicians and patients alike.”
Cardiovascular Business spoke with Reddy at ESC 2023 about the ADVENT trial’s promising results. He emphasized the importance of PFA technology for the future of electrophysiology going forward.
"My own opinion is that PFA will be the workhorse technology we use ... my guess is that once it is approved in the United States, it will likely be utilized quite extensively,” he said.
Boston Scientific has said it plans to launch the newly approved PFA system right away.