FDA clears first pulsed field ablation system to treat AFib
The Food and Drug Administration (FDA) this week cleared a pulsed field ablation (PFA) system, the Medtronic PulseSelect System, for the treatment of both paroxysmal and persistent atrial fibrillation (AF). The approval is the first for this device category in the U.S. It follows the recent European CE mark of the PulseSelect PFA system in November.
"The PulseSelect PFA system ushers the EP community to a new era of safe, effective and efficient AF ablation that overcomes many challenges in our current practice," said Amin Al-Ahmad, MD, clinical cardiac electrophysiologist at St. David's Medical Center in Austin, Texas. St. David's was one of 67 global operators in the PULSED AF trial that led to the approval of the device. "In my clinical experience with the catheter, it was designed for AF ablation procedures. The learning curve in using the catheter and system is short, and the catheter enables the operator to deliver fast and controlled pulsed field energy for AF ablation."
The PulseSelect PFA system is also the first FDA Breakthrough-designated PFA technology to be approved by the FDA. The designation is intended to help patients gain more timely access to medical devices that have the potential to make a significant impact in the diagnosis or treatment of life-threatening conditions.
What is pulsed field ablation and why is it needed?
PFA is widely expected among electrophysiology experts to become the next major trend in arrhythmia care because of its improved safety profile over conventional radio frequency (RF) and cryoablation systems that are the current standard of care.
The new technology delivers short-duration electrical pulses to the cardiac tissue that causes pores, or holes, to form in the cellular walls of the myocytes. This irreversible electroporation process kills the cells without excess heat or cold, which can adversely effect healthy underlying tissues. This has a lot of appeal for electrophysiologists, because existing technologies can damage the esophagus or phrenic nerve, or they can cause pulmonary vein stenosis. Verma said anatomical structures around the right atrium are much more resistant to damage from electroporation. This advance alone makes the technology appealing to electrophysiologists, as it will help avoid these major adverse events.
"What was really impressive was efficiency and the safety. The overall safety event rate was less than 1%. It was just 0.7%, which is one of the lowest complication rates of any AFib ablation trial," explained Atul Verma, MD, director of cardiology with the McGill University Health Centre in Montreal, who presented the late-breaking PULSED-AF pivotal trial outcomes at the American College of Cardiology (ACC) 2023 meeting in March. He explained the details of the trial in an interview with Cardiovascular Business.
"I think over the next five years, we are really going to see pulsed field take up a lot of there thermal ablation space, especially for AFib," he explained. "And I think this being one of the first global, pivotal trial results, it certainly is baring out in that direction."
In terms of efficiency, he said the dwell time in the right atrium was about 60 minutes. This is a far cry from the usual dwell time of a couple hours in traditional AFib ablation procedures.
Overall, Verma said, the success rate of pulsed field ablation was about the same as traditional thermal ablation. About a third of patients did not have their AFib resolved by the therapy during the first attempt procedure. However, he said, the number of repeats procedures was "very low."
"The biggest advance here is the speed and safety," Verma stressed. "Over time, I think we are going to see even better outcomes in terms of efficacy."
"We told operators to not worry too much about overapplying the energy because it is a very safe energy source. We actually encouraged the use of repetitive applications to make sure we got lesions as deep as possible," Verma explained.
Each treatment takes a fraction of a second. Unlike tradition catheter ablation, which requires applying a certain amount of pressure or force to ensure a good scar in the tissue, Verma said, the newly approved device just needs to have contact with the tissue. Contact force is not a factor.
Then the operator rotates the catheter and repeats the therapy to create a lesion that isolates any electrical activity from the pulmonary vein to the rest of the heart. The total application of energy for a full treatment in these patients was less than 30 seconds, Verma said.
"As long as you can visualize that contact with the catheter and the tissue on X-ray or intra-cardiac ultrasound, that is good enough," Verma explained.
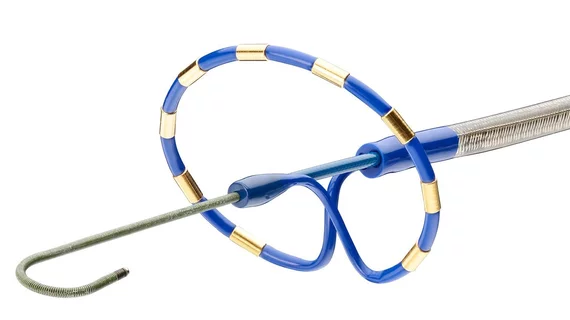
Components of the PulseSelect PFA system
The PulseSelect PFA system also includes the following:
- Designed as a plug-and-play system and can be used with any mapping system or with just fluoroscopy.
- Built-in safety features such as a phrenic nerve test pulse, a non-therapeutic low voltage pulse that provides a preemptive assessment of catheter proximity to the phrenic nerve prior to delivering a therapeutic application.
- Fixed spacing for the nine-electrode catheter, which produces a predictable and consistent electric field for contiguous ablation. In addition to ablation, the nine electrodes can also be used for pacing and sensing.
- The small, 9 French bidirectional catheter enhances maneuverability and access to various anatomical structures and is compatible with a 10 French sheath, including the custom bidirectional FlexCath Contour sheath.
Commercialization of the PulseSelect PFA system will start in early 2024.
Several companies are developing PFA systems. The ADVENT pivotal trial was presented in August as a late-breaking trial at the European Society of Cardiology (ESC). That trial showed positive results for the the Boston Scientific Farapulse PFA system.
Watch the video interview with Verma: Pulsed-field ablation could be the next big EP technology trend.