Medtronic gains 2 key approvals for its ablation portfolio
Medtronic has gained European CE mark approval for its PulseSelect Pulsed Field Ablation (PFA) System and the Nitron CryoConsole.
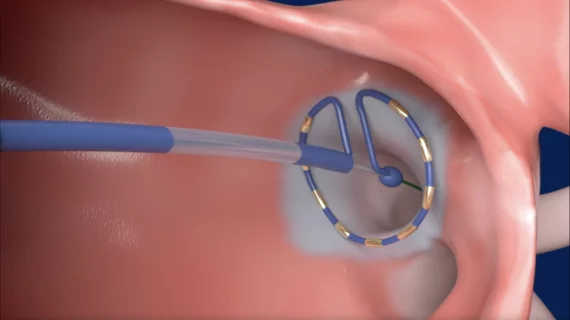
PulseSelect, designed to treat atrial fibrillation (AFib), is a key piece of Medtronic’s cardiac ablation portfolio. It uses PFA to target a patient’s pulmonary veins with 30-second bursts of energy. In trials, pulsed field has the same level of efficacy as traditional thermal ablation using RF or cryo-ablation. However, the safety profile is much greater, because the technology uses energy bursts to create electroporation, where holes developed in cells that lead to cell death, which is easier to control and helps prevent damage to underlying tissues such as the esophagus.
The belief is that PFA helps clinicians target AFib in a way that avoids the risks associated with thermal ablation and cryoablation. Once approved by the U.S. Food and Drug Administration (FDA), many specialists in the cardiology space think it could go on to replace the other ablation modalities for that very reason.
The Nitron CryConsole, meanwhile, houses Medtronic’s Arctic Front and Freezor cryoablation catheters. It includes a new touchscreen interface, wired remote control and features that help guide the operator through ablation procedures.
“With multiple CE mark milestones, today’s announcement demonstrates our commitment to innovation and building a strong electrophysiology portfolio,” Rebecca Seidel, president of Medtronic’s Cardiac Ablation Solutions business, said in a statement. “These milestones are part of our investment in the future of our cryoablation franchise with Nitron, as well as the future of pulsed field ablation with PulseSelect, following our more than ten years of scientific research and development.”
In March 2023, Medtronic gained CE mark approval for its Affera Mapping and Ablation System. According to Medtronic, it is now the first company that offers both single shot and focal PFA options for treating AFib.
The PulseSelect system and the Nitron CryoConsole are not yet approved by the FDA or commercially available in the United States.
November has been a busy month of approvals for Medtronic. The FDA just approved the company’s Symplicity Spyral renal denervation system (RDN) for hypertension, making it just the second RDN system to gain such an approval.