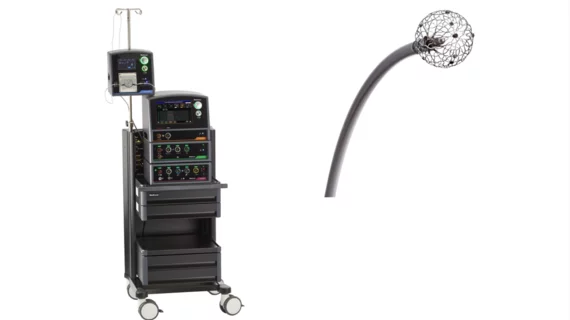
FDA approves Medtronic’s new all-in-one mapping and ablation system for AFib
Medtronic has received U.S. Food and Drug Administration (FDA) approval for its new Affera mapping and ablation system with the Sphere-9 catheter, an all-in-one electrophysiology offering capable of pulsed field ablation (PFA) and radiofrequency (RF) ablation. The system was approved to treat persistent atrial fibrillation (AFib) and cavotricuspid isthmus-dependent atrial flutter. It previously received CE mark approval in March 2023.
Medtronic is now the first company to offer two FDA-approved PFA systems for the treatment of AFib. The company’s PulseSelect PFA system gained FDA approval in December 2023.
The Sphere-9 catheter at the heart of this system includes a 9 mm lattice tip designed to deliver wide-area circumferential ablations. It can provide either pulsed field or RF energy, a feature that puts more treatment options at the fingertips of operators than other commercially available PFA offerings.
“The significance of this innovative technology should be underscored; Affera is a game changer for treatment of AFib and atrial flutter,” Vivek Reddy, MD, director of cardiac arrhythmia services for the Mount Sinai Health System in New York City and a known leader in ablation technologies, said in a statement. “The Affera system provides physicians with one safe, effective and efficient solution to this common and increasing problem in heart disease that needs optimized solutions for patients. With a short learning curve for experienced physicians, the possibilities are boundless for the treatment of AFib.”
“At Medtronic, we have a 75-year tradition of bringing disruptive innovation to market, guided by our mission and commitment to address the unmet needs of patients,” added Rebeca Seidel, president of Medtronic’s cardiac ablation solutions business. “With the approval of Affera, we are excited to bring a novel mapping and ablation solution to clinicians that is intended to make AFib treatment safer, more effective, and more efficient.”
Affera system linked to positive patient data
The FDA’s decision was based largely on data from the SPHERE PER-AF clinical trial, which compared treating persistent AFib with the Affera mapping and ablation system and Sphere-9 catheter to other commercially available treatment options. That study confirmed that the Affera and Sphere-9 technologies were non-inferior to the “conventional standard of care” when patients present with persistent AFib resistant to anti-arrhythmic drugs.
Initial findings from SPHERE PER-AF were presented at the Heart Rhythm Society 2024 annual meeting in Boston and simultaneously published in Nature Medicine.[1]
Click here for an exclusive video interview Cardiovascular Business performed with Devi G. Nail, MD, a cardiologist with St. Bernards Healthcare and co-author of SPHERE PER-AF, when those data were first announced.
Announcement follows Medtronic’s 2022 acquisition of Affera for approximately $925M
This announcement is years in the making for Medtronic. The company acquired Affera, a Massachusetts-based healthcare technology company focused on the treatment of arrhythmias, in August 2022. The deal was reportedly worth $925 million, though the companies never shared the final figures. The two companies were already quite familiar with one another; Medtronic had a 3% ownership in Affera well before this transaction had ever been announced.
“The acquisition enhances and accelerates our ability to treat millions of patients around the world suffering from cardiac arrhythmia with our innovative technology,” Doron Harlev, Affera’s founder and Medtronic’s current vice president of engineering for cardiac ablation solutions, said at the time. “Our team designed the Affera platform with physicians and patients in mind, to advance the field of electrophysiology while supporting safe and efficient cardiac ablation procedures.”