First patients treated with Abbott’s pulsed field ablation offering
Abbott announced this week that more than 30 patients in Australia were treated with the company’s new pulsed field ablation (PFA) system, representing the first time the technology has been used to treat arrhythmias such as atrial fibrillation in a clinical setting.
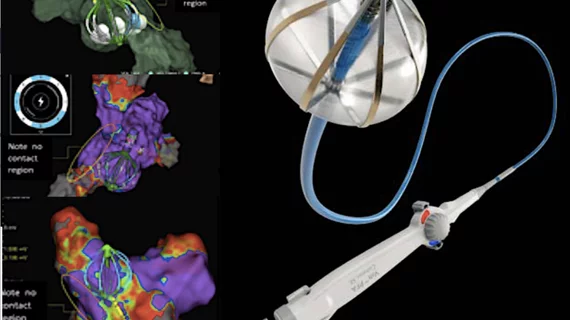
The procedures were performed by Prash Sanders, MBBS, PhD, director of the Center for Heart Rhythm Disorders at the University of Adelaide in Australia. They are the first steps of a new CE mark study designed to evaluate the safety and effectiveness of Abbott’s Volt PFA System.
According to Abbott, the Volt system was specifically designed to address weaknesses seen in other first-generation PFA offerings. It was paired with the company’s EnSite X EP heart mapping, technology for example, a move intended to make PFA procedures more accurate and limit the number of necessary ablations.
“Daily life for the millions of people with AFib can be difficult as symptoms often include palpitations, shortness of breath, dizziness and chest pain, making it critical that physicians treat the issue as soon as possible,” Christopher Piorkowski, MD, chief medical officer of Abbott's electrophysiology business, said in a prepared statement. “With AFib cases expected to rise continuously, Abbott's Volt PFA System meets a growing demand for a more innovative solution that reduces the patient procedure time and overall hospital stay, getting them back to living a fuller, longer life.”
“We have long known that pulsed field ablation could open up an entirely new frontier in how we treat people battling the most complex cardiac arrhythmias,” added Sanders. “But like any innovation, early solutions have not been able to fully capitalize on those potential benefits. Abbott has designed a novel PFA solution that, when combined with its EnSite X cardiac mapping system, can address hard-to-treat irregular heartbeats with a level of accuracy and precision that's never before been possible.”
The Volt PFA System is not presently approved by the FDA. Abbott hopes to gain approval for a clinical trial in the United States in the near future.
Rising momentum for pulsed field ablation
In December, the U.S. Food and Drug Administration (FDA) approved Medtronic’s PulseSelect PFA System for the treatment of AFib. Boston Scientific and Biosense Webster are also among other vendors currently working on developing their own PFA systems.
Electrophysiologists and other cardiology professionals have been expecting PFA to rise in importance for quite some time now. Because it uses electrical fields as opposed to the extreme heat seen with radiofrequency ablation and extreme cold seen with cryoablation, PFA represents a treatment option for patients with AFib and other abnormal heart rhythms that could be much safer and lead to fewer complications.