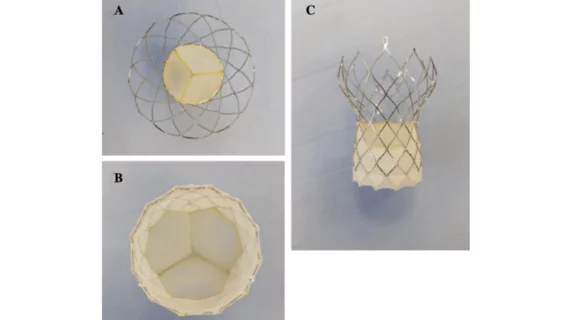
New TAVR valve, premounted to save cardiologists time, linked to promising 30-day outcomes
Interventional cardiologists have performed transcatheter aortic valve replacement (TAVR) with a new self-expandable, supra-annular valve on 10 patients for a first-in-human study, and presented their findings in the American Journal of Cardiology.[1]
The Vienna Aortic Self-Expandable Transcatheter Valve was developed by P+F Products + Features GmbH, a healthcare technology company based out of Vienna, Austria. It arrives fully premounted on a delivery system—a step designed to save cardiologists time—and comes in four sizes: 23, 26, 29 and 31 mm. It was also built to allow resheathing or complete recapture/redeployment if necessary.
“Numerous TAVR devices have been introduced over the years, each with their unique features and advantages,” wrote first author Kasparas Briedis, MD, MSc, with the department of cardiology at the Lithuanian University of Health Sciences in Lithuania, and colleagues. “However, there are currently no fully premounted TAVR devices available on the market. Therefore, the safety and efficacy of these new devices must be evaluated before they can be widely adopted in clinical practice.”
For the VIVA first-in-human study, Briedis et al. treated 10 patients with the Vienna device between November 2021 to June 2022. All patients underwent TAVR with transfemoral access. The mean age of 79 years old, and six patients were men. The group included nine high-risk patients and one intermediate-risk patient. One patient has previously undergone coronary artery bypass graft surgery.
The study’s technical success rate was 100%. Resheathing was required during three procedures and was successful each time with no complications. The mean procedure time was 45.1 minutes. One patient did experience cardiac tamponade as a result of perforation caused by the temporary pacemaker lead, but their condition was stabilized. Another patient experienced a disabling ischemic stroke immediately following the procedure, and they were treated with an emergency mechanical thrombectomy. “No residual neurological deficit” was observed.
After 30 days, the team added, survival was 100%. Also, no myocardial infarctions, acute kidney injuries or major vascular complications were reported, and no patients were hospitalized for valve-related symptoms or congestive heart failure. A second patient did experience a minor stroke five days after the procedure. According to a neurologist who saw the patient, the “underlying cause of persistent neurological deficit at 30 days was a combination of prolonged postoperative recovery and subacute stroke.”
Significant improvements were seen in the mean aortic valve gradient, peak velocity, aortic valve area and effective orifice area index after 30 days. Severe aortic regurgitation (AR) and paravalvular leak (PVL) was not seen in any patients, though moderate AR and moderate PVL were both seen in one patient with severely calcified aortic annulus anatomy.
Significant improvements were also seen in quality of life questionnaires taken before the procedure and then again 30 days following the procedure. This included an improved mean six-minute walk distance.
“The results of this study demonstrated the early procedural safety of the device in a patient population with intermediate or high surgical risk, as evidenced by a 0% mortality rate within 30 days,” the authors wrote. “The observed rate is comparable with that of first-generation and second-generation devices, and the low procedural mortality rates for third-generation balloon-expandable and self-expandable aortic valves implanted through the transfemoral route.”
The team also emphasized that the complication rate seen during this study was comparable to what has been reported for similar devices. The two strokes were noteworthy, but Briedis and colleagues noted that strokes have always been a considerable risk associated with TAVR.
“Importantly, no association was found between patients who underwent re-sheathing of the valve and incidence of any stroke,” the group added.
Another key takeaway from the team’s analysis was the benefit of the premounted valve.
“In comparison with other TAVR devices on the market, the average implantation time was considerably shorter,” the authors wrote. “This could be attributed to the device's exceptional feature of being fully premounted on the delivery system in the manufacturing facilities, eliminating the need for valve assembly and crimping during the procedure, thus reducing the time required for the implantation. Finally, given the fact that this was a first-in-human experience associated with an operator's early learning curve, the average reported procedure time might be overstated and can be reduced after getting more expertise with the Vienna TAVR system.”
While much more research on this valve is still required, the authors praised its efficiency and ability to minimize the steps required to complete a TAVR procedure. The group also noted that, thanks to these advantages, it could prove to be a valuable resource for “developing countries facing logistical and economical challenges.”
Read the full study here. This device has not been approved by the U.S. Food and Drug Administration.