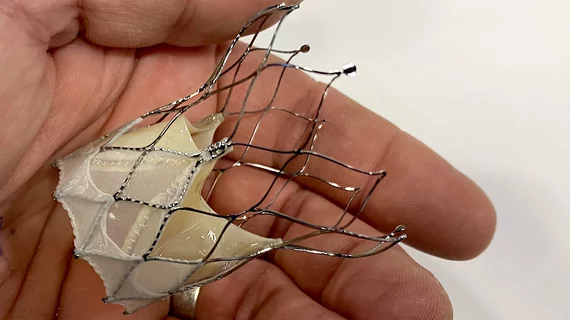
Self-expanding TAVR system with new sealing cuff linked to high success rate, favorable outcomes
Abbott’s Navitor transcatheter aortic valve replacement (TAVR) system, designed with a new-look sealing cuff for limiting paravalvular regurgitation (PVR), is associated with positive 30-day outcomes, according to a new analysis published in Heart, Lung and Circulation.[1]
The self-expanding TAVR valve gained U.S. Food and Drug Administration approval in January 2023 and European CE mark approval in May 2021.
Due to the “novelty” of some of the device’s new features, the study’s authors aimed to provide an updated look at its overall effectiveness. They tracked data from 60 consecutive patients treated with the Navitor TAVR system at a single facility from 2021 to 2022. While the mean patient age was 79 years old, 57% were women and the mean Society of Thoracic Surgeons score was 4.87.
“The cohort presented is reflective of an intermediate-risk to high-risk group with a variable array of valve anatomies treated at an academic heart valve center,” wrote first author Ganeev Malhotra, MBBS, with Princess Alexandra Hospital in Brisbane, Australia, and colleagues. “This cohort includes patients with bicuspid aortic valve disease in addition to patients with degenerated surgical aortic valve replacement prostheses, patients with annular and LVOT calcification, and patients requiring alternative access approaches.”
Overall, after 30 days, TAVR procedures performed with the Navitor device were associated with a procedural success rate of 100% and no patient deaths. There was a single major vascular complication and two non-disabling strokes, but no disabling strokes or transient ischemic attacks. Four patients did require a new permanent pacemaker. No heart failure hospitalizations were reported through 30 days.
Diving deeper into the data, no patients had more than mild PVR, and 75% of patients had “trivial or no PVR.” The mean post-TAVR effective orifice area was 1.95 cm2.
“The clinical application of the Navitor transcatheter heart valve system in this as-treated analysis of consecutive patients outside of a clinical trial setting was associated with high rates of overall procedural success,” the authors wrote. “Post-procedure hemodynamic parameters were favorable, and there appeared to be a low frequency of significant adverse events.”
Abbott did not fund this analysis or help design it in any way.
“The study is an organically investigator-initiated evaluation, and the vendor for the transcatheter heart valve prosthesis used had no role in the study initiation, study design, manuscript preparation or decision to publish,” the authors wrote.
The group did note that following patients for “12 months and longer” would provide a much more complete picture of the valve’s effectiveness. Follow-up data for 10 years, meanwhile, would be required to “elucidate meaningful data around prosthesis durability.”
Read the full study here.