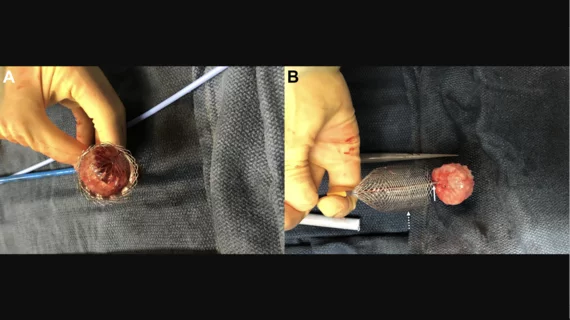
Cardiologists are first in world to remove unstable Watchman devices with FDA-cleared retrieval system
Interventional cardiologists in Houston used a new-look retrieval device to remove unstable left atrial appendage occlusion (LAAO) devices from three different elderly patients. The team wrote about its experience in JACC: Clinical Electrophysiology, saying it was the first time the device had ever been used for that specific purpose.[1]
“Embolization of LAAO devices is associated with significant morbidity and mortality,” wrote first author Daniel Hermann, MD, a cardiologist with Memorial Herman Health System in Houston, and colleagues. “Data from the National Cardiovascular Data Registry LAAO Registry demonstrated that surgical retrieval was frequently required for LAAO device embolization and was associated with a significant risk of mortality. In addition, percutaneous retrieval of embolized/malpositioned LAAO devices can be challenging, particularly when attempting to retrieve these devices into large-bore sheaths.”
Hoping to improve care for these patients, Hermann et al. turned to the ŌNŌ retrieval system, a catheter-delivered device cleared by the U.S. Food and Drug Administration in 2022. The device was developed by Ōnōcor, a U.S. healthcare startup with roots in the Penn Center for Innovation.
“The ŌNŌ retrieval system has been used in different scenarios to date to remove a variety of cardiac devices and large balloon fragments,” the group wrote. “It offers potential advantages over existing retrieval techniques used for removing embolized/malpositioned LAAO devices and may facilitate safer and more effective percutaneous retrieval.”
Three patients over the age of 80 years old were treated using this technique.
The first patient, an 83-year-old man with coronary artery disease (CAD) and chronic atrial fibrillation (AFib), received a 27-mm Watchman FLX device developed by Boston Scientific. On the day the patient was going to be discharged, his care team noticed the device has embolized to his left ventricular outflow tract. The patient underwent a cardiac CT scan and a transesophageal echocardiography, and then the retrieval system was used in the cath lab to remove the device.
The second patient was an 82-year-old man with a history of paroxysmal AFib, chronic systolic congestive heart failure, CAD, hyperlipidemia, hypertension, peripheral artery disease and stroke. He received a 31-mm Watchman FLX device, but it was unstable; cardiologists removed the device with no complications.
The third and final patient, an 81-year old woman, presented with a history of chronic AFib. She received a 31-mm Watchman FLX device, but it was found to be partially dislodged after six weeks and there was a residual leak. The ŌNŌ retrieval system was once again used to remove the device.
Click here for the full breakdown in JACC: Clinical Electrophysiology. The authors included additional details, images and video.