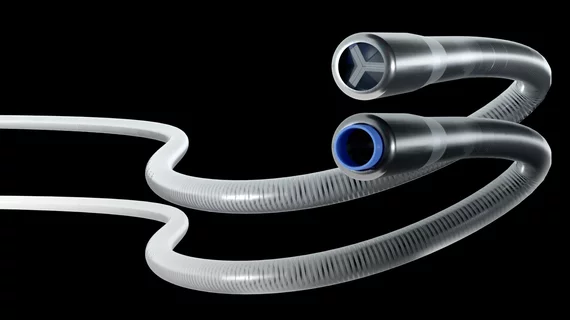
FDA clears new catheter for peripheral blood clots
Expanse ICE, a new healthcare technology company born out of the Expanse Medical medical device incubator, has received U.S. Food and Drug Administration (FDA) clearance for its new-look catheter to treat blood clots in the peripheral arteries and veins.
The ICE Aspiration System features a dual-lumen design that allows it to perform a type of cyclic aspiration at the distal end of the catheter. The company calls this its Surge Aspiration technology.
“We've engineered the ICE catheter system to harness the aspiration power typical of a large bore catheter, but within the slender profile of a much smaller device,” Eitan Konstantino, PhD, founder and CEO of Expanse Medical, said in a statement announcing the FDA’s decision.
“It is clear this device was built with physicians in mind,” added Michael Lichtenberg, MD, chief medical officer of the angiology department Vascular Center Clinic in Arnsberg, Germany. “It aims to address some of the biggest issues we see regularly. The device was designed and built with a strong understanding of foundational physics that leaves me optimistic for its success.”
Expanse Medical is the brainchild of Konstantino, a serial entrepreneur with years of experience in the medical device space. The incubator includes an in-house team of engineers focused on developing new technologies they hope can “disrupt” the current healthcare landscape.