Some weight loss patients are overdosing on semaglutide, FDA warns
The U.S. Food and Drug Administration (FDA) is warning healthcare providers and patients alike that dosing errors associated with some injectable semaglutide products have resulted in multiple adverse events.
Semaglutide products include Novo Nordisk’s Wegovy, the first weight loss drug approved by the FDA to minimize heart risks, and Ozempic. These GLP-1 agonists have received significant attention in recent months for their potential to help overweight and obese patients lose weight, and now the FDA is emphasizing the importance of following recommended dosing information at all times.
Compounded semaglutide drugs linked to significant health risks
Compounded semaglutide injectable products are at the heart of these ongoing concerns. When drugs are compounded, it means they have been combined, mixed or altered in some way that creates a separate medication tailored to a single patient’s needs. While the FDA has indicated that compounded drugs “can serve an important medical need for patients,” the agency does not approve these new-look formulations, meaning they may be associated with additional risks.
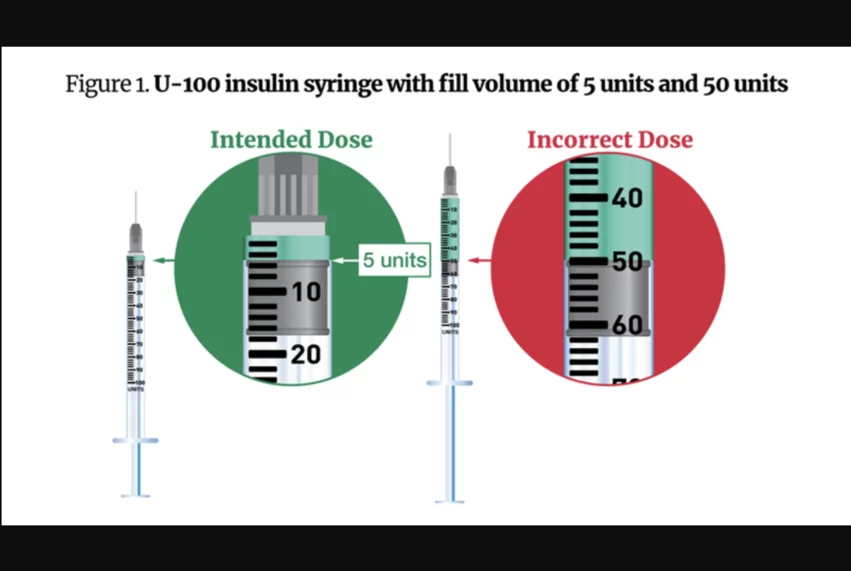
In the case of these compounded semaglutide medications, the FDA warned that errors have been seen in the way patients measure and self-administer the drugs and in how healthcare providers calculate doses.
“FDA encourages patients to talk with their healthcare provider or compounder about how to measure and administer the intended dose of compounded semaglutide,” according to the agency’s warning. “Many of the patients who received vials of compounded semaglutide lacked experience with self-injections, according to the adverse event reports. Unfamiliarity with withdrawing medication from a vial into a syringe and coupled with confusion between different units of measurement may have contributed to dosing errors.”
The adverse effects seen so far include nausea, severe vomiting and severe hypoglycemia.
“A prolonged period of observation and treatment for overdose symptoms may be necessary due to the long half-life of semaglutide of about one week,” the FDA said.