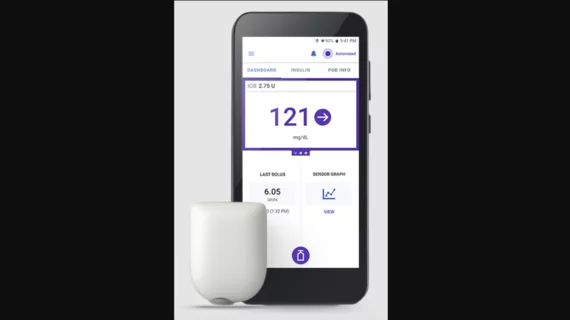
FDA clears first automated insulin pump for type 2 diabetes
Insulet Corporation, a Massachusetts-based medical device company focused on diabetes technology, announced that its Omnipod 5 Automated Insulin Delivery System is now cleared by the U.S. Food and Drug Administration (FDA) to manage type 2 diabetes (T2D). The device was already cleared to manage type 1 diabetes (T1D), and this makes Omnipod 5 the first automated insulin pump cleared by the FDA to manage patients with both T2D and T1D.
“Today’s announcement represents a significant milestone in providing easy-to-use, patient-centric technology for the treatment of T2D,” Jim Hollingshead, Insulet president and CEO, said in a statement. “Insulet is paving the way for these individuals to achieve better health outcomes while living with greater confidence and freedom through the game-changing benefits of tubeless Pod therapy. Omnipod 5 is setting a new standard in diabetes management, and we are thrilled with the opportunity to make a lasting impact on the insulin-requiring T2D community.”
The FDA’s decision to approve this updated indication was based largely on data from SECURE-T2D, a clinical study first presented at the American Diabetes Association’s 84th Scientific Sessions in Orlando. SECURE-T2D compared treatment with the Omnipod 5 device to traditional therapies such as insulin injections or pump therapy. Overall, researchers found that Omnipod 5 appeared to significantly reduce HbA1c, time in hyperglycemia and total daily insulin dose while improving time in range, all without increasing the time patients spend in hypoglycemia.
“Omnipod 5 makes it easier for people with T2D to take their insulin and stay in range, leading to remarkable improvements in clinical outcomes and overall quality of life,” Anne L. Peters, MD, director of the University of Southern California Westside Center for Diabetes, said in the same statement. “I believe this innovative technology has the potential to transform the lives of insulin-requiring people with T2D.”