FDA clears new device for valve-in-valve TAVR patients at risk of coronary obstruction
Pi-Cardia, an Israel-based healthcare technology company, has received U.S. Food and Drug Administration (FDA) clearance for its ShortCut device designed to help interventional cardiologists perform valve-in-valve transcatheter aortic valve replacement (TAVR) on high-risk patients.
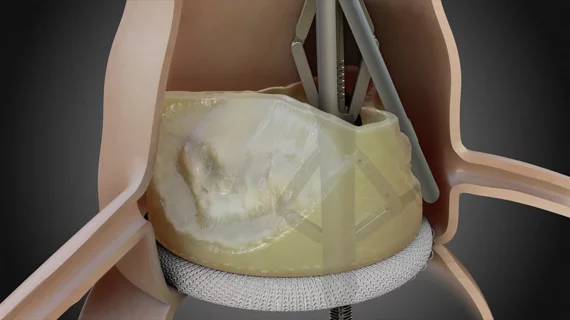
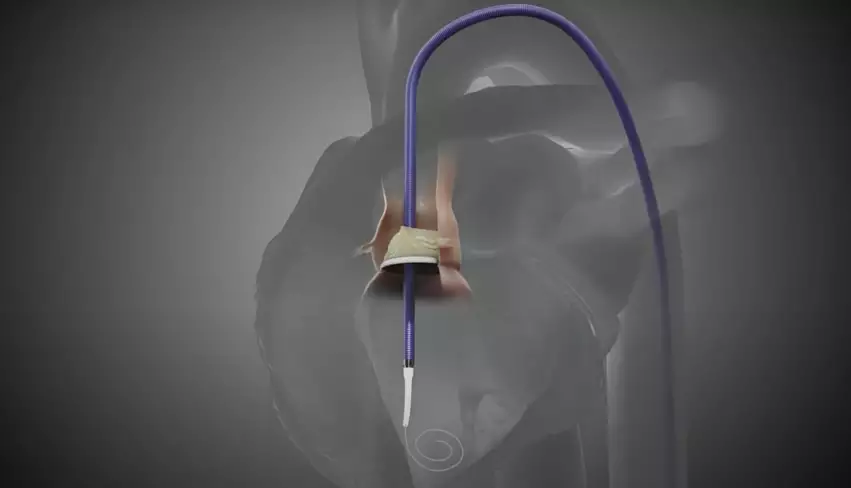
ShortCut, described by Pi-Cardia as the “world’s first dedicated leaflet modification device,” is used to split valve leaflets when patients present with an increased risk of coronary obstruction.
Philippe Genereux, MD, an interventional cardiologist with Morristown Medical Center in New Jersey, and colleagues treated the first U.S. patient with ShortCut in December 2022. The device then received the FDA’s breakthrough device designation in January 2024.
“The rigorous pivotal study leading to this important market clearance by the FDA demonstrates that ShortCut was both safe and effective in achieving the intended leaflet split in all patients,” Martin B. Leon, MD, director of the Center for Interventional Vascular Therapy at New York-Presbyterian/Columbia University Medical Center and founder of the Cardiovascular Research Foundation, who chairs the steering committee for ShortCut clinical studies, said in a statement. “Importantly—it also shows that mechanical splitting with ShortCut, in both single- and dual-leaflet cases, was a controlled and teachable procedure, making it adoptable by TAVR centers as a critical step pre-implantation, so that patients at risk of coronary obstruction may be safely treated, without disruption of TAVR workflow.”
“We are excited and immensely proud to introduce the first dedicated leaflet modification device to the U.S. market,” added Erez Golan, Pi-Cardia CEO. “With FDA clearance now in hand, we are focused on building a strong commercial and clinical support team to execute a limited commercial launch while ensuring optimal patient outcomes.”
Pi-Cardia is also developing the ShortCut Mitral for treating high-risk transcatheter mitral valve replacement patients, and Leaflex, which mechanically scores heart valve calcification.