Surgical devices recalled after 17 serious injuries—FDA warns of possible shortage
The U.S. Food and Drug Administration (FDA) has announced that Maquet Cardiovascular, a subsidiary of Getinge, is recalling certain endoscopic vessel harvesting (EVH) devices due to significant safety concerns. In addition to alerting healthcare providers and medical device distributors about this issue, the agency also wanted to warn that this may lead to an interrupted supply of EVH devices throughout the United States.
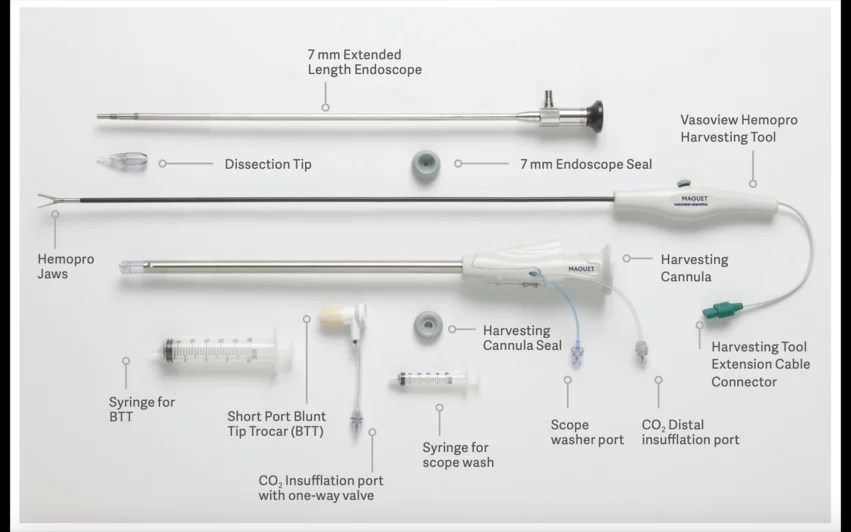
Maquet’s VasoView HemoPro 1.5 and VasoView HemoPro 1 EVH devices are designed to harvest blood vessels from the patient’s body that are then used during arterial bypass procedures such as coronary artery bypass graft (CABG) surgery. However, there have been multiple reports of silicone parts breaking off during the harvesting process. In some cases, the debris could not be recovered.
There have been 17 reports of serious injury so far due to this ongoing issue.
“Detachment of silicone into the patient can lead to an EVH procedural delay and/or conversion of the EVH procedure to a more invasive open vessel harvest procedure,” the FDA said in its advisory.
The FDA is aware of additional incidents involving the VasoView HemoPro 2 devices, but it is still evaluating the issue.
Devices should be returned immediately
The FDA urged healthcare providers to read the full recall notice from Maquet and Getinge. All lots included in the recall are listed on that notice, and any devices from those lots should be returned immediately.
There may be certain situations where healthcare providers have access to no other devices and need to use a recalled VasoView HemoPro device during a cardiac surgery. In those cases, the FDA said special care should be taken to inspect the device for signs of damage or any “rough surfaces, sharp edges or unusual protrusions.” The device should then be watched closely when used.
FDA shares shortage concerns
As a result of these issues, EVH devices have been officially added to the FDA’s medical device shortage list.
“The FDA will continue to work with health care providers and facilities to assist with challenges related to available options for EVH devices,” according to the agency. “The FDA is working with Getinge/Maquet and other manufacturers to identify potential strategies to help support availability of these critical devices.”