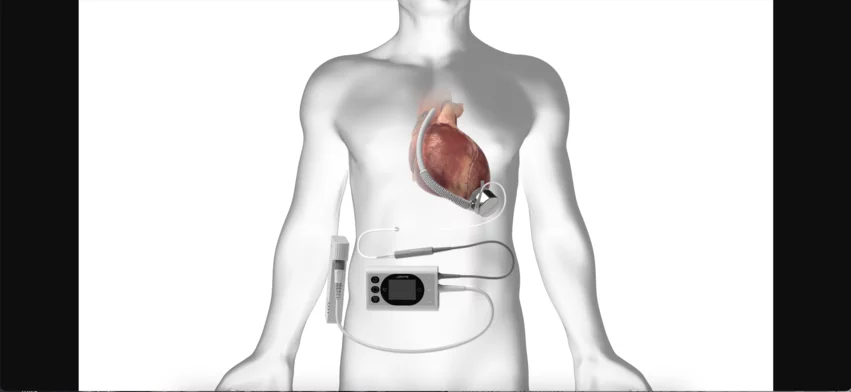
Surgeons make history with first US implant of new magnetically suspended heart pump
Cardiothoracic surgeons at Emory University Hospital have implanted the BrioVAD System, a new type of ventricular assist device (VAD) from BrioHealth Solutions, for the very first time in the United States.
The successful procedure represents the start of the INNOVATE clinical trial, a new non-inferiority study designed to track the safety and effectiveness of the BrioVAD System when treating end-stage heart failure compared to other therapies already on the market. Other participating facilities include Cleveland Clinic, Duke University, and the University of Chicago.
At the center of the BrioVAD System sits the BrioVAD pump, a magnetically suspended, hemocompatible blood pump built to reduce the risk of adverse events. BrioHealth has been developing this advanced technology since 2008. More than 300 patients outside the United States have already been treated with the device.
“We’re honored to be the first implanting site in this study,” Mani Daneshmand, MD, a professor and director of thoracic transplant and mechanical circulatory support surgery with the Emory University School of Medicine, said in a statement. “The BrioVAD is a promising heart assist pump, designed from the ground up with focus on patient safety and quality of life. With this study, we continue our long history of innovation in pursuit of our mission to bring compassionate, quality care to every patient who arrives at our doors seeking care.”
“We are thrilled to kick off the INNOVATE trial, following a phenomenal journey of innovation, engineering, and quality refinement to bring the BrioVAD System to life,” Chen Chen, PhD, CEO of BrioHealth Solutions, said in a separate statement. “Despite advancements in ventricular assist devices, there remains a pressing need for improved device performance and patient outcomes, and BrioHealth is committed to addressing this gap. It is also incredibly rewarding to see the enthusiasm from our participating centers in advancing heart failure treatment through this study.”
Facilities participating in INNOVATE will focus on both short- and long-term outcomes, following patients for a full two years after treatment.
The BrioVAD System has not yet been approved by the U.S. Food and Drug Administration.