First surgical device of its kind granted key FDA exemption
Artivion, an Atlanta-based medical device company, has received a Humanitarian Device Exemption (HDE) from the U.S. Food and Drug Administration (FDA) for its AMDS Hybrid Prosthesis designed to treat DeBakey Type 1 dissections when malperfusion occurs. This HDE ensures select patients can be treated with the device before the FDA makes its final approval decision.
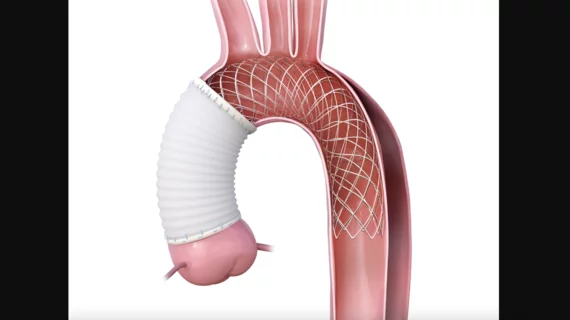
According to Artivion, the AMDS is the first aortic arch remodeling device designed for the treatment of acute DeBakey Type 1 aortic dissections. It was built with a rapid deployment in mind and makes minimally invasive reinterventions possible, reducing the use of more invasive arch repair procedures.
If the AMDS does go on to gain FDA approval, Artivion noted, it would likely cover all acute DeBakey Type 1 dissections, even those without any signs of malperfusion.
The FDA’s decision to grant a HDE was based largely on data from the PERSEVERE US investigational device exemption study. Initial results show that using the AMDS device was linked to a significant reduction in major adverse events, including all-cause mortality and new disabling strokes, compared to traditional treatments.
“The fact that the FDA has recognized the AMDS device through the HDE pathway is very encouraging and speaks to the unique aspects of the device to treat a rare and emergent condition,” Wilson Szeto, MD, chief of cardiovascular surgery at the University of Pennsylvania’s Perelman School of Medicine, said in a statement. “The compelling results from the PERSEVERE study paired with the ease of use and approachability of the AMDS device will undoubtedly expand the ability of all cardiac surgeons to offer a more comprehensive treatment for patients.”
“This HDE from the FDA validates the groundbreaking nature of AMDS, a device with no comparable clinical alternative,” added Pat Mackin, chairman, president and CEO of Artivion. “We will now work diligently with facilities and physicians in the U.S. to expand access to this life saving device as we continue to work towards PMA approval, which we still expect in late 2025. We thank every PERSEVERE investigator and study participant for helping to advance this revolutionary technology.”
Artivion working to bounce back from cyberattack
Artivion experienced a “cybersecurity” incident on Nov. 21, taking systems offline as necessary to avoid the risk of damage, according to recent communication with the U.S. Securities and Exchange Commission.
“As of the date of this filing, we believe that the incident has not had a material impact on the company’s overall financial condition or results of operations and that the incident is not reasonably likely to have a material impact on its financial conditions or results of operations,” the company wrote. “The company continues to provide its products and services to customers, but the incident has caused disruptions to some order and shipping processes, as well as to certain corporate operations, which have largely been mitigated. The company has and will continue to incur expenses related to its response to this incident, and the company believes it has adequate insurance coverage.”
Cardiovascular Business did reach out to Artivion for comment on this cybersecurity issue, but he company has not yet replied.