Hydra TAVR valve gains key approval as global reach continues to grow
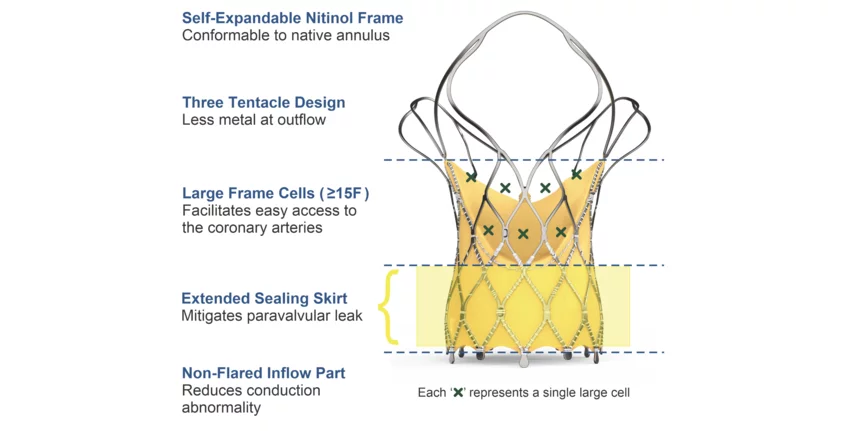
Sahajanand Medical Technologies (SMT), a global medical device company based out of India, has announced that its Hydra transcatheter aortic valve replacement (TAVR) valve is now available in Mexico. The self-expanding device, designed specifically to provide easy access to the coronary arteries, is available in more than 20 countries and even received CE mark approval back in 2020. However, it has not yet been approved by the U.S. Food and Drug Administration for use in the United States.
“With the approval of Hydra in Mexico, we are empowering physicians with an advanced TAVR system that combines precision, safety, and superior hemodynamics,” Joao Rodrigues, SMT’s head of Latin America, said in a prepared statement. “This milestone underscores our commitment to saving lives and transforming cardiac care in Latin America.”
The Hydra valve was originally developed and launched by Vascular Concepts, which SMT acquired in 2020 when it first entered the structural heart disease market. SMT acquired Vascular Innovations, another medical device company known for structural heart disease offerings, that same year.
In 2022, an analysis published in JACC: Cardiovascular Interventions, an American College of Cardiology journal, explored one-year outcomes in patients treated with the Hydra TAVR valve.[1] Tracking data from 157 patients with a mean age of 79.2 years old, researchers found that that the Hydra device was associated with a device success rate of 82.8%, all-cause mortality rate of 14.6% and “significant improvements” in both effective orifice area and mean aortic valve gradient.
The group called for additional research into the safety and effectiveness of the Hydra device, specifically in low-risk patients and those who present with bicuspid aortic valves.