New research highlights rising interest in LBBAP
There has been an explosion of interest the past few years in left bundle branch area pacing (LBBAP) as an emerging technique to improve ventricular pacing support by providing more physiologic activation of cardiac tissue than conventional pacing locations. Several EP device vendors now have leads with new indications for LBBAP from the U.S. Food and Drug Administration (FDA).
This week, Biotronik enrolled its first patient in the second arm of the BIO-CONDUCT study at NYU Langone Health. The FDA investigational device exemption (IDE) trial is examining the use of Biotronik's next-generation Solia CSP S pacing lead specifically aimed at LBBAP. The company said the Solia CSP S is designed with a fixed helix for simpler preparation, a lengthened helix for ease of LBBA access, and an optimized distal end for high durability.
The first arm of the study between 2022 and 2024 looked at the safety and efficacy of the Solia S for LBBAP. The second arm of this study that just launched will look at the new version of the device optimized for LBBAP.
“As we continue to refine the LBBAP technique in the lab, we need equally refined tools that will lead to successful patient outcomes. The Solia CSP S lead builds on the excellent outcomes we saw with Solia S in BIO-CONDUCT and other studies, and will continue to assist physicians in providing more patients with access to this beneficial procedure,” Larry Chinitz, MD, director of cardiac electrophysiology with the NYU Langone Heart Rhythm Center and co-director of NYU Langone Heart, said in a statement.
Chinitz and Lior Jankelson, MD, PhD, director of the Inherited Arrhythmia Program at NYU, performed the first procedure Monday.
The second arm of the IDE trial seeks to demonstrate the safety and effectiveness of the investigational pacing lead when used in the left bundle branch area. It will enroll up to 110 patients at up to 14 sites in the United States. Successful lead implant and complication rates, as well as lead performance and quality of life, will be assessed over 12 months.
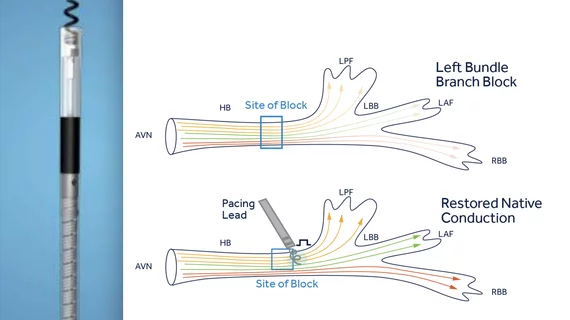
Vendors moving toward LBBAP
Several vendors are involved in LBBAP trials and working toward new device approvals or an expansion of FDA indications for LBBAP. Medtronic was the first to gain a new LBBAP indication for its SelectSecure MRI SureScan Model 3830 cardiac lead in October 2022. It originally gained FDA approval for His-bundle pacing in 2018.
In September 2024, the FDA cleared three more new LBBAP indications. Boston Scientific received the indication for its Ingevity+ pacing leads, Biotronik received the new indication for its Solia S lead and Selectra 3D catheter and Abbott's UltiPace stylet-driven pacing lead was also cleared for LBBAP use.
In December 2024, Abbott announced the world's first in-human leadless LBBAP procedures using the company's investigational Aveir Conduction System Pacing (CSP) leadless pacemaker system. The procedures were part of a feasibility study and were performed at Na Homolce Hospital in Prague, Czech Republic, and Mount Sinai Hospital in New York. The FDA granted Breakthrough Device Designation for the Aveir CSP for LBBAP.
"While both conduction system pacing and leadless pacing provide distinct benefits to many patients, they have been separate options – until now," said Devi Nair, MD, director of cardiac electrophysiology at St. Bernards Medical Center in Jonesboro, Arkansas, in a statement. Abbott said she has been a key contributor to the study.
Growing movement in EP labs toward LBBAP
Left bundle branch pacing, also referred to as conduction system pacing, was a big topic at the 2023 Heart Rhythm Society meeting with two big late-breaking studies. Read more about these studies. The interest was also very evident in sessions at Heart Rhythm 2024, and at a packed evening dinner session where experienced operators explained details on how to perform LBBAP lead placements.
Rather than using two leads to pace each ventricle in left bundle branch block (LBBB) patients, a single lead can be placed in the LBB located in the septum after the area of block. While this can simplify pacemaker procedures and reduce the amount of hardware put inside a patient, there are some challenges with the anatomy and exact location of where top place a lead that can be more complex.
"It's a little bit more difficult probably for many patients who have relatively good heart muscle function. It probably doesn't make that much difference whether you pace the septum or whether you pace the septum and get their conduction system captured. But in patients who have heart failure, it does make a big difference," explained HRS President Kenneth Ellenbogen, MD, director of clinical cardiac electrophysiology and pacing for Virginia Commonwealth University, in an interview on EP trends with Cardiovascular Business.
LBB pacing can perform even better than cardiac resynchronization therapy (CRT) in most cases, Ellenbogen added.
“Conduction system pacing is more like simulating natural activation and can yield positive outcomes for patients,” said Pugazhendhi Vijayaraman, MD, director of electrophysiology at the Geisinger Heart Institute, said in a statement after the FDA approval of Medtronic's LBBAP lead in 2022. He said the FDA's approval of that first indication would encourage more physicians to learn the procedure, which has happened and has increased with addition vendor approvals.
Conduction system pacing is being heavily researched as an alternative to traditional coronary sinus pacing.
"Most of LBB pacing has been used for the pacing indication of AV block, and we started using it as a first-line therapy in patients with heart failure and had excellent results with it. We think it is a move forward in the treatment of patients with heart failure," Juan C. Diaz, MD, a specialist with Clinica Las Vegas in Medellin, Colombia, explained to Cardiovascular Business in an interview in 2023. He presented the results from the SYNCHRONY Collaborative Group late-breaking trial at HRS 2023, which compared LBBAP vs. standard biventricular pacing (BiVp) for CRT.
"The results from our study were excellent. We had a significant 38% decrease in the composite outcome of heart failure, hospitalization and all-cause mortality. And this impact was seen very early on from the beginning of the study," he explained. He said heart failure symptoms also decreased, with approximately 80% of patients seeing an improvement of at least one functional NYHA heart failure class, compared to just 66% in the BiVp arm of the study.
The success of this and the other trials has continued to fuel interest in LBBAP.