Medtronic cardiac lead the first to receive FDA approval for conduction system pacing
Medtronic announced on Monday, Oct. 17, that one of its cardiac leads has been approved by the U.S. Food and Drug Administration (FDA) for conduction system pacing.
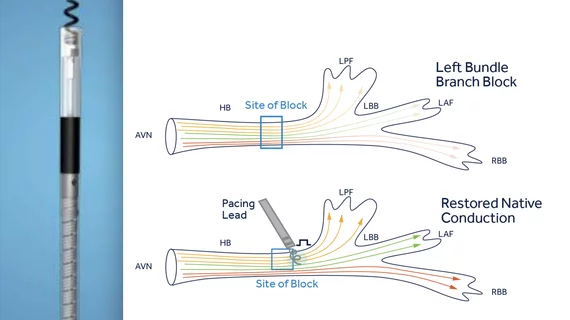
The company’s SelectSecure MRI SureScan Model 3830 cardiac lead already gained FDA approval for His-Bundle pacing in 2018. Now, the lead is also approved for left bundle branch area pacing, providing users with another option when treating patients for bradycardia. According to Medtronic, this marks the first time the FDA has approved a device for this indication.
Pugazhendhi Vijayaraman, MD, director of electrophysiology at the Geisinger Heart Institute in Wilkes-Barre, Pennsylvania, said in a prepared statement that the approval should help more cardiology professionals embrace the potential of conduction system pacing.
“Conduction system pacing is more like simulating natural activation and can yield positive outcomes for patients,” Vijayaraman said. “This approval signals to physicians that the Model 3830 lead is safe and effective for patients for conduction system pacing, and it may encourage more physicians to learn the procedure.”
This new approval is based on data from more than 20,000 patients. Use of the SelectSecure Model 3830 cardiac lead for left bundle branch area pacing has been associated with a success rate of 92% and complication rates of approximately 2.5%.
“Physicians are telling us about their excitement for the future of pacemakers, which will rely on conduction system and leadless pacing,” Robert C. Kowal, MD, PhD, Medtronic’s general manager of cardiac pacing therapies and an experienced electrophysiologist, said in the same statement. “Expanded labeling of this lead allows us to train physicians to successfully perform left bundle procedures, bringing the benefits of conduction system pacing to more patients.”
Like all MRI SureScan pacing leads developed by Medtronic, the SelectSecure Model 3830 is safe to be included in cardiac MRI scans when necessary.