Class I recall issued for Abbott’s HeartMate 3 heart pump
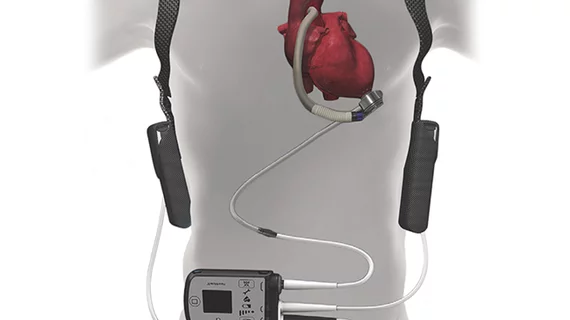
The FDA has issued a Class I recall of the HeartMate 3 Left Ventricular Assist System (Abbott) because a malfunction in the device’s outflow graft assembly could lead to graft occlusion, possibly reducing or stopping blood flow.
This occurrence may result in blood clots and death, according to the FDA’s statement on the recall, which affects 4,878 units in the U.S. distributed since September 2014. The HeartMate system is designed to provide circulatory support for patients with end-stage left ventricular heart failure and includes an outflow graft that connects a blood pump to the aorta.
Occlusion of the outflow graft would trigger a low-flow alarm in the device. The FDA said patients experiencing this alarm should contact their physicians immediately, although Abbott doesn’t recommend removing the device because of this issue.
Abbott began contacting physicians last month about the potential problem with the heart pumps. Thirty-two incidents of outflow graft twisting—including three related patient deaths—had been reported prior to the company issuing a press release on May 4. Abbott sent specific instructions to physicians for managing patients on May 21 as part of an urgent recall notification, according to the FDA.
The FDA said customers seeking more information can contact Abbott’s 24/7 Mechanical Circulatory Support HeartLine at 1-800-456-1477.