FDA clears AI-assisted CCTA software that assesses plaques for signs of heart disease
Caristo Diagnostics, an Oxford-based medtech company founded by cardiologists, has gained U.S. Food and Drug Administration (FDA) clearance for its CaRi-Plaque technology that uses artificial intelligence (AI) to help detect signs of coronary artery disease (CAD) in routine medical images.
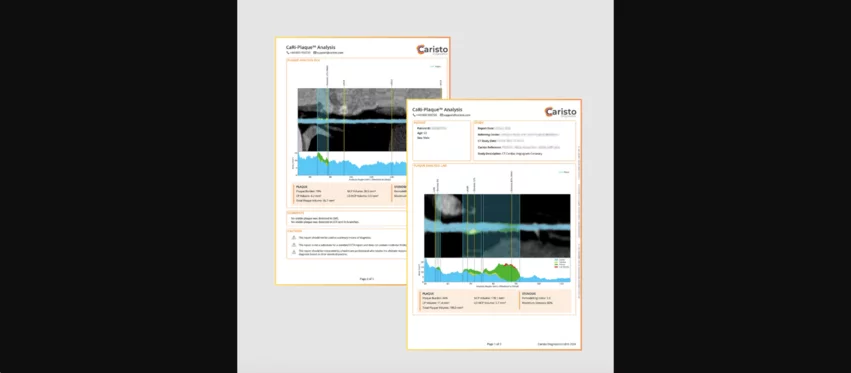
CaRi-Plaque allows clinicians to assess a patient’s coronary health using coronary CT angiography (CCTA) images. It was built to identify and evaluate both coronary plaques and luminal stenosis. CaRi-Plaque reports are reviewed by the company’s trained specialists and then delivered as either a DICOM-conformant file or a PDF.
“For decades, heart disease has been treated reactively, waiting for symptoms to appear before taking action,” Caristo Diagnostics CEO Frank Cheng said in a statement. “But with AI, we can change that. With FDA clearance for CaRi-Plaque, hospitals and clinics can now move beyond traditional diagnostics and into truly proactive, personalized heart attack prevention.”
Another AI-powered offering for heart patients to keep an eye on
An additional Caristo Diagnostics offering, CaRi-Heart, gained CE mark approval to be marketed and sold in Europe in 2021, but technology has not been fully cleared by the FDA. CaRi-Heart was built to evaluate CCTA images for signs of coronary inflammation and provide clinicians with in-depth risk scores.
When it does gain approval, the company noted, CaRi-Plaque users will be able to seamlessly adopt CaRi-Heart and then take advantage of both tools at once to evaluate a patient’s heart disease risks in CCTA images.
Additional insights into Caristo Diagnostics
Cardiovascular Business spoke with Charalambos Antoniades, MD, PhD, co-founder and chief scientific officer of Caristo Diagnostics, at the American Heart Association’s 2024 annual meeting. He provided an in-depth look at the company’s technologies, detailing how imaging focused on inflammation could prove to be game-changer for cardiologists and heart patients alike.
Click here for the full video interview.
CCTA keeps building momentum
Cardiac CT has been on the rise for quite some time, providing care teams with a noninvasive way to evaluate heart patients for a long list of potential complications. In late 2024, the U.S. Centers for Medicare and Medicaid Services even finalized a new payment policy that more than doubled the Medicare reimbursements hospitals receive for performing CCTA exams.
“We’re thrilled with the CMS’s ruling, which better aligns with the cost of providing CCTA services,” Ahmad Slim, MD, chair of the SCCT Health Policy and Practice Committee, said at the time. “This is a huge win for U.S. providers as well as the entire cardiac imaging community, ultimately improving patient access to this essential diagnostic tool, which aligns with the society’s overall mission.”
CCTA’s rise has continued to be a major story to watch in 2025. The FDA’s clearance of CaRi-Plaque is yet another sign that this modality is becoming more important to today’s cardiologists.