Leadless pacemaker technology moves toward dual-chamber pacing
When the first catheter-implanted, leadless pacemaker was introduced in 2016, there was a lot of interest in this minimally invasive technology. But the biggest criticism was that it only addressed the needs of about 20% of patients. However, 100% of patients requiring pacemakers may soon have a leadless option, based on data being collected in an ongoing clinical trial assessing the performance of two leadless pacemakers in different chambers of the heart that can communicate with each other.
The Medtronic Micra was the first leadless pacemaker cleared by the FDA in 2016. The Abbott Aveir VR became the second in March 2022. However, both are single-chamber pacemakers, which limits their use to just 20% of patients requiring pacing, while 80% require dual-chamber pacing.
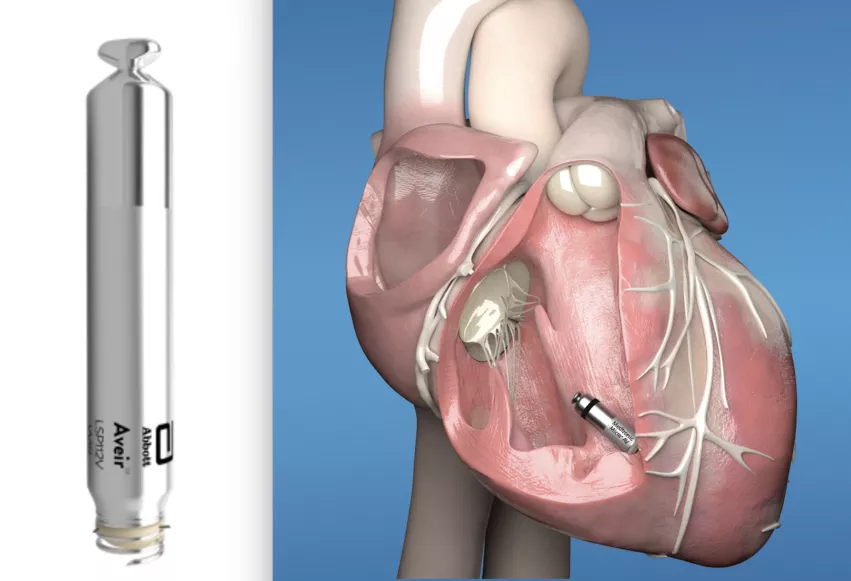
This may soon change, with the ongoing Abbott Aveir DR i2i clinical study, which is assessing the use of a leadless pacemaker implanted into two chambers of the heart that can communicate with each other to establish synchrony. The software for Aveir VR Leadless Pacemaker was designed for expandability to a dual chamber system to be introduced to the Aveir VR system via software updates in the future, after regulatory approval.
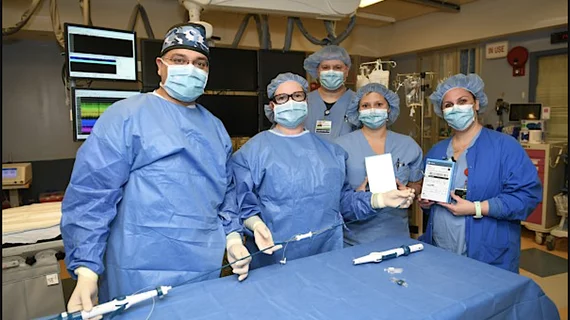
“This is incredibly significant as nearly 80% of people who receive a pacemaker need a dual-chamber option to pace both chambers on the right side of the heart,“ explained Electrophysiologist Mark Mascarenhas, MD, Hackensack Meridian Jersey Shore University Medical Center, in a statement from the hospital. “While leadless pacemakers work like traditional pacemakers to regulate heart rate, they offer reduced lead-related complications and a less restrictive recovery period due to the minimally invasive implant procedure. This technology is a great enhancement in what we can provide to our patients with abnormal heart rhythms.”
The cardiovascular team at Hackensack Meridian Jersey Shore University Medical Center recently implanted New Jersey’s first dual-chamber leadless pacemaker systems in patients, as part of the Aveir DR i2i clinical study.
“Providing this new system is a landmark moment for people requiring assistance to maintain a healthy heart rhythm,” said Mascarenhas.
He explained leadless pacing options have been limited to single-chamber devices until now since synchronization of two leadless pacemakers has been highly difficult to achieve. There has been anticipation for the past several years that these smaller types of pacemakers could coordinate pacing in different chambers if they can communicate with each other, but it has taken years to develop the technology enough for use in human trials.
Abbott designed its i2i technology to provide beat-by-beat communication between two leadless pacemakers, one positioned in the right ventricle and one positioned in the right atrium of the heart. This helps regulate the heart rate synchronously between chambers allowing for dual-chamber leadless pacing.
Medtronic gained approval for its AV synchrony leadless pacing system
In January 2020, Medtronic received FDA clearance for its leadless Micra atrioventricular (AV) synchrony device. The technology uses a single leadless pacemaker that can monitor the pacing from the atrium and send pulses to match that rhythm in the ventricle.
The Micra AV is indicated for the treatment of AV block, which occurs when the electrical signals between a person’s atria and ventricles are impaired. Its approval was based on positive results from the MARVEL 2 study, which evaluated the ability of the Micra’s internal sensor to monitor and detect atrial contractions and achieve AV synchrony between the heart’s chambers. The trial met its primary efficacy endpoint, finding that a significantly greater portion of complete heart block patients with normal sinus rhythm achieved 70% or greater AV synchrony during algorithm-mediated pacing.
When is a pacemaker needed?
People who experience a slower-than-normal heart rate (bradycardia) might need a pacemaker to regulate their heart beat. Conventional pacemakers use a small battery-powered device implanted in the chest wall under the skin that delivers electrical impulses via thin insulated lead wires that are connected to the pacemaker and routed through veins into the heart. The leads cause the heart muscle chambers to contract to help restore a normal heart rhythm.
Unlike traditional pacemakers, leadless pacemakers are implanted directly into the heart through a minimally invasive catheter-based procedure. This eliminates the need for cardiac leads in the veins and for surgical incisions to implant the larger pacemaker "can" under the skin.
Nearly 2 million Americans have a pacemaker implantation according to Medtronic.
Pacemaker battery longevity is a key factor in pacemaker implants, as the device will need replacement when the battery nears the end of its life. Abbott says its Aveir VR Leadless Pacemaker has a battery life of about 10 years. Medtronic says the Micra has a battery life of between 8-13 years depending on pace needs.
Related Leadless Pacemaker Content:
First implant of dual-chamber leadless pacemaker completed in Abbott’s Aveir DR i2i pivotal trial
Leadless devices reduce infections in pacemaker-dependent patients
Abbott’s single-chamber leadless pacemaker gains FDA approval
FDA highlights safety issue with leadless pacemakers, focusing on Medtronic’s Micra
FDA approves world’s smallest pacemaker with AV synchrony
Implantable pacemaker market slated to top $5.3B by 2023
VIDEO: 4 predictions on key cardiac technologies for the coming years