Medtronic’s next-generation leadless pacemakers gain FDA approval
Medtronic has received U.S. Food and Drug Administration approval for its next-generation Micra leadless pacemakers, the Micra AV2 and Micra VR2 transcatheter pacing systems.
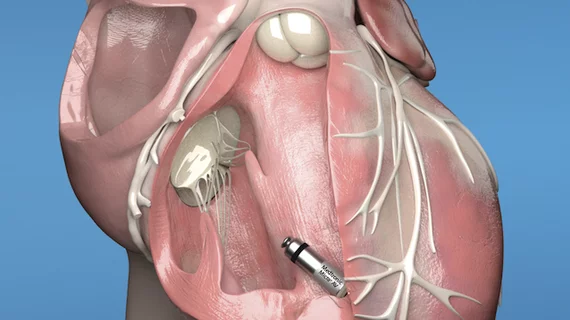
The original Micra leadless pacemaker gained FDA approval in 2016. It created significant industry attention for its small size—Medtronic regularly describes it as “the world’s smallest pacemaker”—and the fact that is implanted inside the patient’s heart rather than in a surgical pocket under the armpit.
These next-generation Micra pacemakers have much longer battery life than previous models. The Micra AV2 has a median battery life of nearly 16 years, and the Micra VR2 has a median battery life of nearly 17 years. In addition, advanced artificial intelligence algorithms built into the Micra AV2 help it automatically program AV synchrony, and its higher tracking capability was designed with more active patients in mind.
Both pacemakers are implanted directly into the heart via a minimally invasive procedure; no chest incision is needed to implant these devices.
“Our goal is to improve the patient experience by continuously reinventing our ground-breaking leadless pacemaker,” Robert C. Kowal, MD, PhD, general manager of cardiac pacing therapies within Medtronic’s Cardiac Rhythm Management business, said in a prepared statement. “Since inventing the first battery-operated cardiac pacemakers 65 years ago, Medtronic has transformed pacing technologies to benefit patients, including the nearly 200,000 patients globally who have received a Micra device so far.”