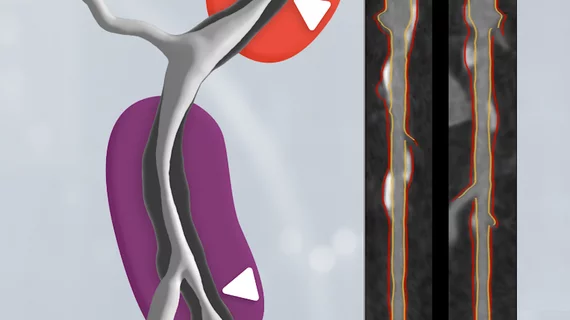
HeartFlow gains FDA clearance for 2 new AI-powered imaging assessments
HeartFlow has received U.S. Food and Drug Administration (FDA) clearance for two of its new artificial intelligence (AI) solutions, Plaque Analysis and RoadMap Analysis.
Both use coronary CT angiography (CCTA) to provide clinicians with a noninvasive look at patients who present with coronary artery disease (CAD) and face a heightened myocardial infarction risk. While Plaque Analysis is focused on delivering a detailed analysis of any plaque found in the patient’s coronary arteries, RoadMap Analysis was developed for the identification and diagnosis of stenosis. The company has already found success with its HeartFlow FFR-CT Analysis solution, which captures noninvasive anatomical and physiological assessments of a patient’s coronary artery anatomy using fractional flow reserve CT (FFR-CT) imaging.
“It is exciting to note the work HeartFlow is doing to bring forward the innovative technologies to help us advance our understanding and care for patients with CAD,” Jagat Narula, MD, PhD, chief of cardiology at Mount Sinai Morningside Hospital, said in a prepared statement from HeartFlow. “Combining anatomy, physiology, and plaque morphology would be essential for personalized patient care.”
John Farquhar, president and CEO of HeartFlow, called this moment “a major milestone in the company’s commitment to provide physicians with richer clinical insights to help diagnose and treat individual patients, no matter where they are on the coronary disease spectrum.”
“FFR-CT has already been recognized by the recent ACC/AHA Chest Pain Guidelines and is poised to change the standard of care in patients,” he added. “Plaque and RoadMap analyses, together with FFR-CT, establish HeartFlow’s platform technologies and will enable further development of AI-powered risk scoring to better identify asymptomatic patients at risk of heart attack.”
According to HeartFlow, its next step with these newly cleared technologies involves acquiring real-world data from patients at a select group of U.S. hospitals and health systems.