Flurpiridaz data shows promise to expand and enhance cardiac PET
The biggest news from the American Society of Nuclear Cardiology (ASNC) 2022 meeting was positive late-breaking data on the phase 3 Aurora trial for the flurpiridaz (F-18) positron emission tomography (PET) radiotracer agent. Numerous nuclear cardiology experts have predicted the radiopharmaceutical might significantly accelerate PET adoption in nuclear cardiology with its 106 minute half life and more precise imaging that SPECT.
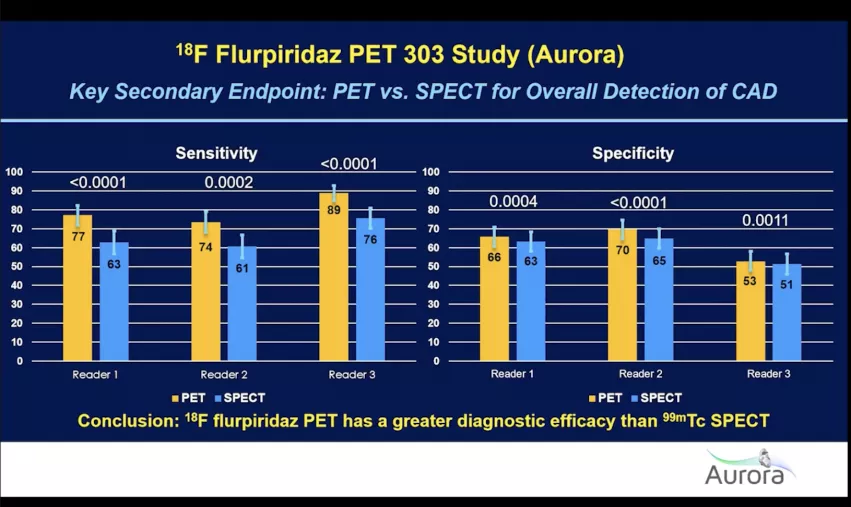
Flurpiridaz PET was found to be clearly superior in all areas assessed compared with more commonly used SPECT nuclear imaging. The results of the phase 3, open-label, multicenter study of flurpiridaz injection for PET imaging assessment of myocardial perfusion (MPI) in patients referred for invasive angiography because of suspected coronary artery disease (CAD), the Aurora study, found much greater sensitivity and specificity than SPECT in all areas and types of patients compared.
"Flurpiridaz has a greater diagnostic efficacy that technetium labeled SPECT," explained Jamshid Maddahi, MD, FACC, FASNC, health sciences clinical professor, molecular and medical pharmacology, University of California Los Angeles (UCLA). He is the principle investigator for the trial who presented the results at ASNC Sept. 9.
The long-awaited trial results were billed to be one of the most important presentations at this year's ASNC meeting by society President Dennis Calnon, MD, MASNC, Director of Cardiac Imaging for McConnell Heart Hospital at Riverside Methodist Hospital and Director of Nuclear Imaging for OhioHealth Heart and Vascular Physicians.
Maddahi, who has been involved in the development of this radiotracer for about 15 years, said this has been a long journey for flurpiridaz. It started in 2007 with the first in-human testing at UCLA when the product was first developed by Lantheus Medical Imaging. In 2017 the rights to the radiotracer were transferred for GE Healthcare. The completion of the phase 3 trial and its data lock took place in August 2022.
Flurpiridaz has several advantages in PET cardiac imaging compared to Rubidium-82
The flurpiridaz has advantages over the current rubidium-82 (Rb-82) imaging agent that is the current standard of cardiac PET imaging. Rb-82 only has a 75 second half life, so the imaging needs to be done quickly and does not allow for treadmill stress. Flurpiridaz has a long 109 minute half life, allowing production and distribution of the radiotracer by the existing network of regional cyclotrons and the ability to use treadmill stress.
The ability to order from regional cyclotrons enables orders for single doses. Maddahi said this allows lower volume centers to perform PET without the need for a rubidium generator, which requires a higher volume of patients to make the generator and cardiac PET cost effective.
Maddahi also said flurpiridaz has a higher myocardial extraction fraction and a shorter positron range, which offers additional advantages. These include better tracking of blood flow across a broader flow range than other agents, better image resolution and defect contrast, better detection of mild perfusion and better quantification of myocardial blood flow.
The combination of these advantages will likely make cardiac PET much more attractive to centers that have debated about purchasing PET scanners.
"I hope with this agent we have both a practical gateway to doing more PET studies and also being able to offer a better imaging technique than what we already have for evaluating coronary artery disease," Maddahi explained.
This hope that flurpiridaz will help enable the expansion of coronary PET was echoed by Sharmila Dorbala, MD, MPH, Director of Nuclear Cardiology, Brigham and Women 's Hospital, Professor of Radiology, Harvard Medical School, and past ASNC president.
"This is really exciting for the nuclear imaging community, because for sites that do not have a current cardiac PET program, you can now see the emergence of cardiac PET perfusion imaging in labs that were not previously doing perfusion imaging. The results are so exciting and the big question is now if when can we get this approved by the FDA."
Maddahi said the data will be presented to the FDA for a final regulatory review. If all goes well, he said that review could come by the end of 2023.
"We hope this will be approved soon by the FDA and be introduced into the clinical setting," said George Beller, MD, MASNC, Professor at the University of Virginia Health System, and moderator of the late-breaking session.
Flurpiridaz Aurora trial details presented at ASNC 2022
The primary endpoint of the trial included assessing the diagnostic efficacy of flurpiridaz injection PET MPI for detecting significant CAD (50% or greater CAD). The secondary endpoint was measurement of the diagnostic efficacy of flurpiridaz compared with SPECT MPI in detecting CAD. The trial included 46 international clinical sites.
The study dosed 604 patients with flurpiridaz, and included 587 in a SPECT evaluation arm, 588 in an invasive angiography arm, and 578 who received PET, SPECT and angiography. Of these patients, 43% were found to have CAD. The imaging was reviewed by three double blinded expert readers.
About 52% had BMI of more than 30. "In the 298 patients with BMI equal to or greater than 30, we can again see flurpiridaz has a greater diagnostic efficacy than SPECT," Maddahi reported.
The study also separated out results from 194 diabetic patients. Maddahi said this is a population that is growing and there was a lot of interest in understanding if flurpiridaz could enhance imaging in these patients. "Again, flurpiridaz PET had a greater diagnostic efficacy that SPECT," Maddahi said.
The overall image quality was also judged, and the percentage of studies was determined to be excellent or good. "All three readers found significantly better image quality for PET compared to SPECT," Maddahi said.
In an assessment of diagnostic certainty, again PET scored much better than SPECT, with readers saying they had a much higher level of confidence in their interpretation using 18F flurpiridaz.
The comparison of ischemic defect extent and severity was also boasted to be much better using PET vs. SPECT. "There is a significantly larger ischemic defect severity extent detected on PET compared to SPECT.
"On average, there were 75% larger defects on PET compared to SPECT," Maddahi said.
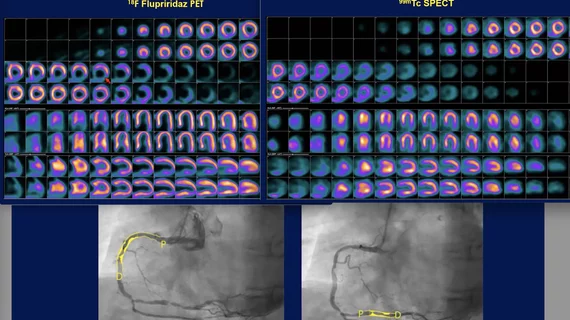
He showed several patient examples from the trial. One patient had a normal SPECT, but the flurpiridaz PET imaging showed clear ischemic defects that matched a 54% and 67% blockage assessed by coronary angiography. Other patients also showed normal SPECT scans but clearly identifiable perfusion defects on the flurpiridaz PET imaging.
Safety of F-18 flurpiridaz
In the Aurora trial, there were 25 adverse events that were judged to be possibly related to flurpiridaz. In 14 patients, the adverse events were classed as serious and clinically significant. But, there were not clinical lab changes from baseline reported in these patients. Also, there were no changes in the patients' ECG.