Radiology AI company gains FDA clearance for new CT offerings focused on PE, stroke
Avicenna.AI, a French artificial intelligence (AI) startup co-founded by a radiologist, has received U.S. Food and Drug Administration (FDA) clearance for two new offerings designed to automatically identify cardiovascular findings in CT scans, CINA-iPE and CINA-ASPECTS.
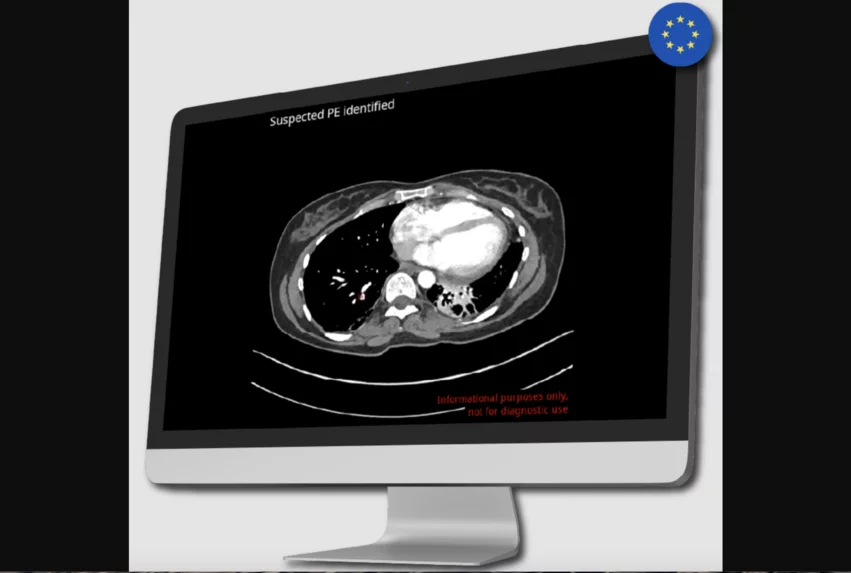
CINA-iPE is an advanced algorithm trained to detect incidental signs of pulmonary embolism (PE) in routine CT scans. It can evaluate a variety of different scan times, including full-body and cardiac CT, and it alerts the user whenever a PE is identified. The model’s performance was validated with imaging data from 381 CT scans performed for reasons other than evaluating the patient for PE; it was able to work with imaging results captured with 39 different scanner models.
CINA-ASPECTS, meanwhile, is an advanced algorithm trained to quantify stroke severity after reading non-contrast CT scans of the brain. The AI model provides immediate insights when used, automatically calculating the patient’s ASPECT (Alberta Stroke Program Early CT) score. According to Avicenna.AI, ASPECT scores can be widely different one radiologist to the next; one of the primary reasons the company wanted to develop this offering was to help provide more consistent care from one specialist to the next.
CINA-ASPECTS represents a breakthrough of sorts for Avicenna.AI; it is the company’s first FDA-cleared AI model to land in the computer-aided diagnosis category.
“After a long journey of dedication and perseverance, we are thrilled to announce the FDA clearance of not one, but two of our groundbreaking products,” Cyril Di Grandi, co-founder and CEO of Avicenna.AI, said in a prepared statement. “The clearance of CINA-iPE and CINA-ASPECTS marks a significant milestone in our mission to strive for excellence in advancing patient care. These achievements stand as a testament to the unwavering commitment of our team.”
Avicenna.AI has gained FDA clearance for several AI-powered offerings, including models for intracranial hemorrhage, large vessel occlusion and aortic dissection.