FDA clears AI screening tool for cardiac amyloidosis
Ultromics, a U.K.-based healthcare company focused on using artificial intelligence (AI) to improve echocardiography evaluations, has received U.S. Food and Drug Administration (FDA) clearance for its AI-powered screening software for cardiac amyloidosis.
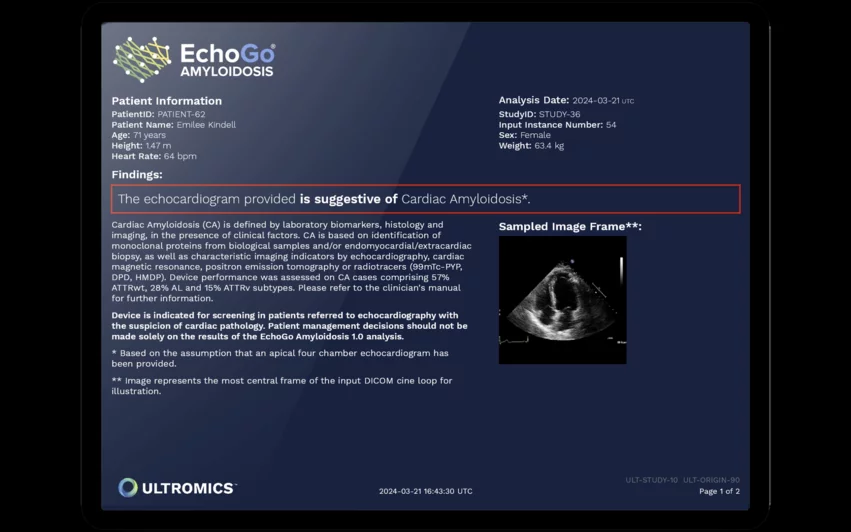
EchoGo Amyloidosis was designed to evaluate routine echocardiogram results for signs of cardiac amyloidosis, alerting clinicians when follow-up care is necessary. The software was developed with assistance from Janssen Biotech, a Johnson & Johnson company, and Pfizer, the company behind multiple FDA-approved cardiac amyloidosis medications. It was previously granted the FDA’s breakthrough device designation in April 2023.
“Echocardiography is a powerful tool for evaluating cardiac structure and function and is central to the detection and monitoring of disease,” Ross Upton, PhD, CEO and founder of Ultromics, said in a statement. “However, there are some diseases that are very challenging for even the most expert clinician to detect on an echocardiogram. Requiring only a single apical four-chamber image, EchoGo Amyloidosis identifies cardiac amyloidosis and will help drive earlier access to appropriate treatment and care for patients with this underdiagnosed disease.”
“Improving the detection of cardiac amyloidosis is vital as early detection provides the greatest therapeutic benefit for patients,” added Sanjiv J. Shah, MD, director of research for the Bluhm Cardiovascular Institute and director of the Center for Deep Phenotyping and Precision Medicine in the Institute for Augmented Intelligence in Medicine at Northwestern University Feinberg School of Medicine. “Novel AI-based diagnostic tools such as EchoGo Amyloidosis from Ultromics should help facilitate disease identification, particularly in clinics and hospitals restricted by expertise and resource. With more therapies becoming available, the FDA approval of EchoGo Amyloidosis is timely.”
EchoGo Amyloidosis is now an official part of the Ultromics EchoGo platform, joining the company’s flagship offering, EchoGo Heart Failure.
More progress for cardiac amyloidosis management after years of little movement
Cardiac amyloidosis is a rare, potentially fatal condition that occurs when abnormal precursor proteins form amyloid fibrils that accumulate in a patient’s heart tissue. Treatment options were limited until 2019, when Pfizer gained FDA approval for tafamidis meglumine and tafamidis, two treatments sold under the brand names Vyndaqel and Vyndamax, respectively. Imaging specialists, especially those who specialize in echocardiography technology, have made identifying the disease a top priority in recent years.