FDA announces new recall of Abbott’s HeartMate LVADs after 14 deaths
The U.S. Food and Drug Administration (FDA) has announced that Abbott and Thoratec, one of its subsidiaries, are recalling nearly 14,000 HeartMate left ventricular assist devices (LVADs) after ongoing safety issues have resulted in multiple deaths. This is a Class I recall, which means the FDA believes using the devices “may cause serious injuries or death.”
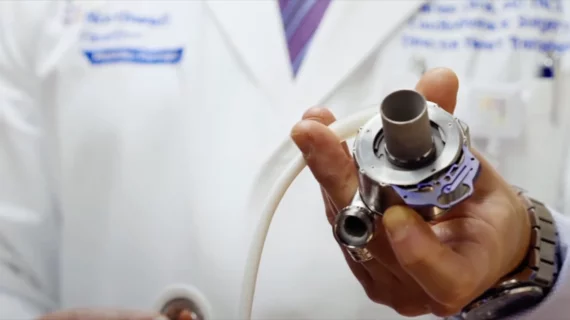
The issue at the center of this recall, which includes both the HeartMate II LVAD and HeartMate 3 LVAD, is the gradual buildup of biological materials within the devices.
“This buildup can obstruct the device, making it less effective in helping the heart pump blood,” according to the advisory. “It can trigger alarms indicating low blood flow and affect the device's ability to help the heart properly. The accumulation of biological material typically occurs over two years or more. The use of affected left ventricular assist system may cause serious adverse health consequences and in worst cases, could result in death.”
A total of 14 deaths and 273 injuries have been linked to these obstructions so far.
The HeartMate 3 LVAD approved by the FDA for the long- and short-term treatment of patients with advanced heart failure. It is the only device of its kind currently available in the U.S. for this vulnerable patient population. It works by pumping blood for the patient’s left ventricle, diverting it to other areas of the body. Cardiologists and cardiac surgeons often turn to these devices before or after a heart transplant. In some cases, the HeartMate 3 LVAD is viewed as a permanent destination therapy when nothing else will work.
This recall is a correction, not a full product removal
Unlike many Class I recalls, the FDA is not urging all impacted devices to be returned to the manufacturer. Instead, customers are advised to pay close attention to low-flow alarms, the first sign that there may be a significant outflow obstruction. An Urgent Medical Device Correction letter sent to customers on Feb. 19 includes more detailed recommendations for addressing such issues as they arise.
"While rates of outflow obstruction are low, Abbott worked to ensure physicians were aware of persistent low flow alarms that can provide an early warning to the potential issue as well as about diagnostic recommendations that can assess any potential obstruction," an Abbott spokesperson told Cardiovascular Business. "The company also reinforced options for treating an outflow obstruction. There are no products being returned as a result of this communication and patients whose LVADs are functioning normally without persistent low flow alarms have no reason for concern."
More details from the FDA on this ongoing issue are available here and here.